Eculizumab Pharmacokinetics and Pharmacodynamics in Patients With Generalized Myasthenia Gravis
- PMID: 34795626
- PMCID: PMC8594444
- DOI: 10.3389/fneur.2021.696385
Eculizumab Pharmacokinetics and Pharmacodynamics in Patients With Generalized Myasthenia Gravis
Abstract
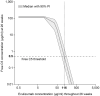
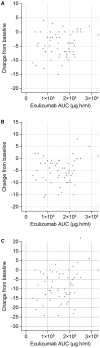
Objective: To investigate the pharmacokinetics, pharmacodynamics, and exposure-response of the approved 900/1,200 mg dosing regimen for the terminal complement component 5 (C5) inhibitor eculizumab in patients with generalized myasthenia gravis (gMG). Methods: The analysis used data from 62 patients aged ≥ 18 years with anti-acetylcholine receptor (AChR) antibody-positive refractory gMG who received eculizumab during the REGAIN study (ClinicalTrials.gov: NCT01997229). One- and two-compartment population-pharmacokinetic models were evaluated, and the impact of covariates on pharmacokinetic parameters was assessed. Relationships between eculizumab exposure and free C5 concentration, in vitro hemolytic activity, clinical response, and tolerability were characterized. Results: Steady-state serum eculizumab concentrations were achieved by Week 4 and were sustained throughout the 26-week treatment period. The eculizumab pharmacokinetic data were well-described by a two-compartment model with first-order elimination, including effects of body weight on pharmacokinetic parameters and plasma-exchange events on clearance. Complete inhibition of terminal complement was achieved in nearly all patients at the time of trough and peak eculizumab concentrations at all post-dose timepoints assessed (free C5 < 0.5 μg/ml in 92% of patients; in vitro hemolysis < 20% in 87% of patients). Serum eculizumab concentrations of ≥116 μg/ml achieved free C5 concentrations of < 0.5 μg/ml. Clinical efficacy and tolerability were consistent across the eculizumab exposure range. Conclusions: Rigorous, quantitative, model-based exposure-response analysis of serum eculizumab concentration and response data demonstrated that the approved eculizumab dosing (900/1,200 mg) for adults with anti-AChR antibody-positive refractory gMG rapidly achieved complete inhibition of terminal complement activation and provided sustained clinical efficacy across the eculizumab exposure range.
Keywords: autoimmune; complement; eculizumab; exposure-response analysis; generalized myasthenia gravis; pharmacodynamics; pharmacokinetics.
Copyright © 2021 Monteleone, Gao, Kleijn, Bellanti and Pelto.
Conflict of interest statement
JM and RP are employed by Alexion Pharmaceuticals, Inc.; XG was employed by Alexion Pharmaceuticals, Inc. at the time the work described in this paper was undertaken; HJK and FB are employed by Certara Strategic Consulting, which received funding from Alexion Pharmaceuticals, Inc.
Figures


References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

