Ets-2 Propagates IL-6 Trans-Signaling Mediated Osteoclast-Like Changes in Human Rheumatoid Arthritis Synovial Fibroblast
- PMID: 34795667
- PMCID: PMC8593237
- DOI: 10.3389/fimmu.2021.746503
Ets-2 Propagates IL-6 Trans-Signaling Mediated Osteoclast-Like Changes in Human Rheumatoid Arthritis Synovial Fibroblast
Erratum in
-
Corrigendum: Ets-2 Propagates IL-6 Trans-Signaling Mediated Osteoclast-Like Changes in Human Rheumatoid Arthritis Synovial Fibroblast.Front Immunol. 2021 Nov 26;12:808756. doi: 10.3389/fimmu.2021.808756. eCollection 2021. Front Immunol. 2021. PMID: 34899765 Free PMC article.
Abstract
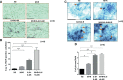
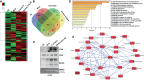
Rheumatoid arthritis synovial fibroblasts (RASFs) contribute to synovial inflammation and bone destruction by producing a pleiotropic cytokine interleukin-6 (IL-6). However, the molecular mechanisms through which IL-6 propels RASFs to contribute to bone loss are not fully understood. In the present study, we investigated the effect of IL-6 and IL-6 receptor (IL-6/IL-6R)-induced trans-signaling in human RASFs. IL-6 trans-signaling caused a significant increase in tartrate-resistant acid phosphatase (TRAP)-positive staining in RASFs and enhanced pit formation by ~3-fold in the osteogenic surface in vitro. IL-6/IL-6R caused dose-dependent increase in expression and nuclear translocation of transcription factor Ets2, which correlated with the expression of osteoclast-specific signature proteins RANKL, cathepsin B (CTSB), and cathepsin K (CTSK) in RASFs. Chromatin immunoprecipitation (ChIP) analysis of CTSB and CTSK promoters showed direct Ets2 binding and transcriptional activation upon IL-6/IL-6R stimulation. Knockdown of Ets2 significantly inhibited IL-6/IL-6R-induced RANKL, CTSB, and CTSK expression and TRAP staining in RASFs and suppressed markers of RASF invasive phenotype such as Thy1 and podoplanin (PDPN). Mass spectrometry analysis of the secretome identified 113 proteins produced by RASFs uniquely in response to IL-6/IL-6R that bioinformatically predicted its impact on metabolic reprogramming towards an osteoclast-like phenotype. These findings identified the role of Ets2 in IL-6 trans-signaling induced molecular reprogramming of RASFs to osteoclast-like cells and may contribute to RASF heterogeneity.
Keywords: interleukin-6; osteoclast; reprogramming; rheumatoid arthritis; synovial fibroblasts.
Copyright © 2021 Singh, Haque, Madarampalli, Shi, Wildman, Basit, Khuder, Prasad, Hassan, Ahmed and Ouseph.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Henderson B, Edwards JCW, Pettipher ER. Mechanisms and Models in Rheumatoid Arthritis. London; San Diego: London; San Diego: Academic Press; (1995).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

