Longitudinal Follow Up of Immune Responses to SARS-CoV-2 in Health Care Workers in Sweden With Several Different Commercial IgG-Assays, Measurement of Neutralizing Antibodies and CD4+ T-Cell Responses
- PMID: 34795668
- PMCID: PMC8593002
- DOI: 10.3389/fimmu.2021.750448
Longitudinal Follow Up of Immune Responses to SARS-CoV-2 in Health Care Workers in Sweden With Several Different Commercial IgG-Assays, Measurement of Neutralizing Antibodies and CD4+ T-Cell Responses
Abstract
Background: The risk of SARS-CoV-2 infection among health care workers (HCWs) is a concern, but studies that conclusively determine whether HCWs are over-represented remain limited. Furthermore, methods used to confirm past infection vary and the immunological response after mild COVID-19 is still not well defined.
Method: 314 HCWs were recruited from a Swedish Infectious Diseases clinic caring for COVID-19 patients. IgG antibodies were measured using two commercial assays (Abbot Architect nucleocapsid (N)-assay and YHLO iFlash-1800 N and spike (S)-assays) at five time-points, from March 2020 to January 2021, covering two pandemic waves. Seroprevalence was assessed in matched blood donors at three time-points. More extensive analyses were performed in 190 HCWs in September/October 2020, including two additional IgG-assays (DiaSorin LiaisonXL S1/S2 and Abbot Architect receptor-binding domain (RBD)-assays), neutralizing antibodies (NAbs), and CD4+ T-cell reactivity using an in-house developed in vitro whole-blood assay based on flow cytometric detection of activated cells after stimulation with Spike S1-subunit or Spike, Membrane and Nucleocapsid (SMN) overlapping peptide pools.
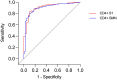
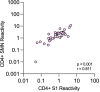
Findings: Seroprevalence was higher among HCWs compared to sex and age-matched blood donors at all time-points. Seropositivity increased from 6.4% to 16.3% among HCWs between May 2020 and January 2021, compared to 3.6% to 11.9% among blood donors. We found significant correlations and high levels of agreement between NAbs and all four commercial IgG-assays. At 200-300 days post PCR-verified infection, there was a wide variation in sensitivity between the commercial IgG-assays, ranging from <30% in the N-assay to >90% in the RBD-assay. There was only moderate agreement between NAbs and CD4+ T-cell reactivity to S1 or SMN. Pre-existing CD4+ T-cell reactivity was present in similar proportions among HCW who subsequently became infected and those that did not.
Conclusions: HCWs in COVID-19 patient care in Sweden have been infected with SARS-CoV-2 at a higher rate compared to blood donors. We demonstrate substantial variation between different IgG-assays and propose that multiple serological targets should be used to verify past infection. Our data suggest that CD4+ T-cell reactivity is not a suitable measure of past infection and does not reliably indicate protection from infection in naive individuals.
Keywords: CD4+ T cells; SARS-CoV-2; antibodies; health care workers; neutralizing antibodies.
Copyright © 2021 Marklund, Leach, Nyström, Lundgren, Liljeqvist, Nilsson, Yilmaz, Andersson, Bemark and Gisslén.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



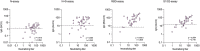

References
-
- World Health Organization . Summary of Probable SARS Cases With Onset of Illness From 1 November 2002 to 31 July 2003 (2015). Available at: https://www.who.int/publications/m/item/summary-of-probable-sars-cases-w... (Accessed July 28, 2021).
-
- World Health Organization . MERS Situation Update (2015). Available at: http://www.emro.who.int/images/stories/csr/documents/mers-cov_15_june.pd... (Accessed October 25, 2021).
-
- Rashid-Abdi M, Krifors A, Sälléber A, Eriksson J, Månsson E. Low Rate of COVID-19 Seroconversion in Health-Care Workers at a Department of Infectious Diseases in Sweden During the Later Phase of the First Wave; a Prospective Longitudinal Seroepidemiological Study. Infect Dis (2021) 53(3):169–75. doi: 10.1080/23744235.2020.1849787 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

