Spectroscopic Signatures of Hydrogen-Bonding Motifs in Protonic Ionic Liquid Systems: Insights from Diethylammonium Nitrate in the Solid State
- PMID: 34795809
- PMCID: PMC8592064
- DOI: 10.1021/acs.jpcc.1c05137
Spectroscopic Signatures of Hydrogen-Bonding Motifs in Protonic Ionic Liquid Systems: Insights from Diethylammonium Nitrate in the Solid State
Abstract
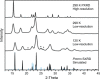
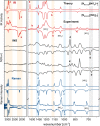
Diethylammonium nitrate, [N0 0 2 2][NO3], and its perdeuterated analogue, [N D D 2 2] [NO3], were structurally characterized and studied by infrared, Raman, and inelastic neutron scattering (INS) spectroscopy. Using these experimental data along with state-of-the-art computational materials modeling, we report unambiguous spectroscopic signatures of hydrogen-bonding interactions between the two counterions. An exhaustive assignment of the spectral features observed with each technique has been provided, and a number of distinct modes related to NH···O dynamics have been identified. We put a particular emphasis on a detailed interpretation of the high-resolution, broadband INS experiments. In particular, the INS data highlight the importance of conformational degrees of freedom within the alkyl chains, a ubiquitous feature of ionic liquid (IL) systems. These findings also enable an in-depth physicochemical understanding of protonic IL systems, a first and necessary step to the tailoring of hydrogen-bonding networks in this important class of materials.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Deetlefs M.; Fanselow M.; Seddon K. R. Ionic Liquids: the View from Mount Improbable. RSC Adv. 2016, 6, 4280–4288. 10.1039/c5ra05829e. - DOI
-
- Plechkova N. V.; Seddon K. R.. Methods and Reagents for Green Chemistry; John Wiley & Sons, Inc., 2007; pp 103–130.
-
- Izgorodina E. I.Ionic Liquids Uncoiled; John Wiley & Sons, Inc., 2012; pp 181–230.
LinkOut - more resources
Full Text Sources
