Role of Fluorination in the Histone Deacetylase 6 (HDAC6) Selectivity of Benzohydroxamate-Based Inhibitors
- PMID: 34795871
- PMCID: PMC8591742
- DOI: 10.1021/acsmedchemlett.1c00425
Role of Fluorination in the Histone Deacetylase 6 (HDAC6) Selectivity of Benzohydroxamate-Based Inhibitors
Abstract
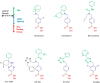
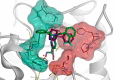
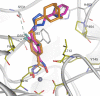
Nonselective histone deacetylase (HDAC) inhibitors show dose-limiting side effects due to the inhibition of multiple, essential HDAC subtypes that can be limited or prevented by restricting their selectivity. We herein report the crystal structures of zebrafish HDAC6 catalytic domain 2 (zHDAC6-CD2) in complex with the selective HDAC6 inhibitors ITF3756 and ITF3985 and shed light on the role of fluorination in the selectivity of benzohydroxamate-based structures over class I isoforms. The reason for the enhancement in the selectivity of the benzohydroxamate-based compounds is the presence of specific interactions between the fluorinated linker and the key residues Gly582, Ser531, and His614 of zHDAC6, which are hindered in class I HDAC isoforms by the presence of an Aspartate that replaces Ser531. These results can be used in the design and development of novel, highly selective HDAC6 inhibitors.
© 2021 American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): Cyprian D. Cukier and Karol Zrubek received financial support from Italfarmaco for the crystallization of ITF3756 and ITF3985.
Figures

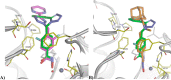
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases

