Mesoporous Bioactive Glasses in Cancer Diagnosis and Therapy: Stimuli-Responsive, Toxicity, Immunogenicity, and Clinical Translation
- PMID: 34796680
- PMCID: PMC8805580
- DOI: 10.1002/advs.202102678
Mesoporous Bioactive Glasses in Cancer Diagnosis and Therapy: Stimuli-Responsive, Toxicity, Immunogenicity, and Clinical Translation
Abstract
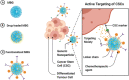
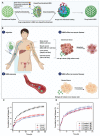
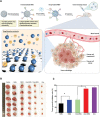
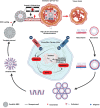
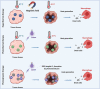
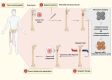
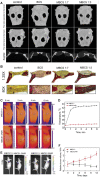
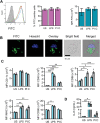
Cancer is one of the top life-threatening dangers to the human survival, accounting for over 10 million deaths per year. Bioactive glasses have developed dramatically since their discovery 50 years ago, with applications that include therapeutics as well as diagnostics. A new system within the bioactive glass family, mesoporous bioactive glasses (MBGs), has evolved into a multifunctional platform, thanks to MBGs easy-to-functionalize nature and tailorable textural properties-surface area, pore size, and pore volume. Although MBGs have yet to meet their potential in tumor treatment and imaging in practice, recently research has shed light on the distinguished MBGs capabilities as promising theranostic systems for cancer imaging and therapy. This review presents research progress in the field of MBG applications in cancer diagnosis and therapy, including synthesis of MBGs, mechanistic overview of MBGs application in tumor diagnosis and drug monitoring, applications of MBGs in cancer therapy ( particularly, targeted delivery and stimuli-responsive nanoplatforms), and immunological profile of MBG-based nanodevices in reference to the development of novel cancer therapeutics.
Keywords: cancer therapy; diagnosis; gene and drug delivery; immunotherapy; mesoporous bioactive glasses; toxicological profile.
© 2021 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













Similar articles
-
Innovative cancer therapy: Unleashing the potential of macromolecule-loaded mesoporous bioactive glasses for precision diagnosis and treatment.Int J Pharm. 2024 Dec 25;667(Pt A):124847. doi: 10.1016/j.ijpharm.2024.124847. Epub 2024 Oct 30. Int J Pharm. 2024. PMID: 39486491 Review.
-
Prevention of bacterial adhesion to zwitterionic biocompatible mesoporous glasses.Acta Biomater. 2017 Jul 15;57:472-486. doi: 10.1016/j.actbio.2017.05.005. Epub 2017 May 5. Acta Biomater. 2017. PMID: 28483701
-
Achievements in Mesoporous Bioactive Glasses for Biomedical Applications.Pharmaceutics. 2022 Nov 29;14(12):2636. doi: 10.3390/pharmaceutics14122636. Pharmaceutics. 2022. PMID: 36559130 Free PMC article. Review.
-
Biomimetic component coating on 3D scaffolds using high bioactivity of mesoporous bioactive ceramics.Int J Nanomedicine. 2011;6:2521-31. doi: 10.2147/IJN.S25647. Epub 2011 Oct 21. Int J Nanomedicine. 2011. PMID: 22072886 Free PMC article.
-
Mesoporous bioactive glasses: Promising platforms for antibacterial strategies.Acta Biomater. 2018 Nov;81:1-19. doi: 10.1016/j.actbio.2018.09.052. Epub 2018 Sep 28. Acta Biomater. 2018. PMID: 30273742 Review.
Cited by
-
Current and Future Perspectives of Bioactive Glasses as Injectable Material.Nanomaterials (Basel). 2024 Jul 13;14(14):1196. doi: 10.3390/nano14141196. Nanomaterials (Basel). 2024. PMID: 39057873 Free PMC article. Review.
-
Nanomaterials: leading immunogenic cell death-based cancer therapies.Front Immunol. 2024 Aug 9;15:1447817. doi: 10.3389/fimmu.2024.1447817. eCollection 2024. Front Immunol. 2024. PMID: 39185425 Free PMC article. Review.
-
Cell loaded hydrogel containing Ag-doped bioactive glass-ceramic nanoparticles as skin substitute: Antibacterial properties, immune response, and scarless cutaneous wound regeneration.Bioeng Transl Med. 2022 Aug 3;7(3):e10386. doi: 10.1002/btm2.10386. eCollection 2022 Sep. Bioeng Transl Med. 2022. PMID: 36176609 Free PMC article.
-
Multi-layer-structured bioactive glass nanopowder for multistage-stimulated hemostasis and wound repair.Bioact Mater. 2023 Feb 11;25:319-332. doi: 10.1016/j.bioactmat.2023.01.019. eCollection 2023 Jul. Bioact Mater. 2023. PMID: 36844363 Free PMC article.
-
Surfactant-Enabled Nanocarriers in Breast Cancer Therapy: Targeted Delivery and Multidrug Resistance Reversal.Pharmaceutics. 2025 Jun 13;17(6):779. doi: 10.3390/pharmaceutics17060779. Pharmaceutics. 2025. PMID: 40574091 Free PMC article. Review.
References
-
- Ashrafizadeh M., Najafi M., Makvandi P., Zarrabi A., Farkhondeh T., Samarghandian S., J. Cell. Physiol. 2020, 235, 9241. - PubMed
-
- Dabbagh Moghaddam F., Akbarzadeh I., Marzbankia E., Farid M., khaledi L., Reihani A. H., Javidfar M., Mortazavi P., Cancer Nanotechnol. 2021, 12, 14.
-
- Moghaddam F. D., Mortazavi P., Hamedi S., Nabiuni M., Roodbari N. H., Anticancer. Agents Med. Chem. 2020, 20, 790. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
