"Porous and Yet Dense" Electrodes for High-Volumetric-Performance Electrochemical Capacitors: Principles, Advances, and Challenges
- PMID: 34796698
- PMCID: PMC8811823
- DOI: 10.1002/advs.202103953
"Porous and Yet Dense" Electrodes for High-Volumetric-Performance Electrochemical Capacitors: Principles, Advances, and Challenges
Abstract
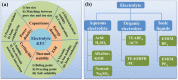
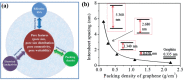
With the ever-rapid miniaturization of portable, wearable electronics and Internet of Things, the volumetric performance is becoming a much more pertinent figure-of-merit than the conventionally used gravimetric parameters to evaluate the charge-storage capacity of electrochemical capacitors (ECs). Thus, it is essential to design the ECs that can store as much energy as possible within a limited space. As the most critical component in ECs, "porous and yet dense" electrodes with large ion-accessible surface area and optimal packing density are crucial to realize desired high volumetric performance, which have demonstrated to be rather challenging. In this review, the principles and fundamentals of ECs are first observed, focusing on the key understandings of the different charge storage mechanisms in porous electrodes. The recent and latest advances in high-volumetric-performance ECs, developed by the rational design and fabrication of "porous and yet dense" electrodes are then examined. Particular emphasis of discussions then concentrates on the key factors impacting the volumetric performance of porous carbon-based electrodes. Finally, the currently faced challenges, further perspectives and opportunities on those purposely engineered porous electrodes for high-volumetric-performance EC are presented, aiming at providing a set of guidelines for further design of the next-generation energy storage devices.
Keywords: electrochemical capacitors; high volumetric performance; portable and wearable electronics; “porous and yet dense” electrodes.
© 2021 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures















Similar articles
-
Toward Superior Capacitive Energy Storage: Recent Advances in Pore Engineering for Dense Electrodes.Adv Mater. 2018 Apr;30(17):e1705713. doi: 10.1002/adma.201705713. Epub 2018 Mar 14. Adv Mater. 2018. PMID: 29537115 Review.
-
Electrochemical Capacitors with Confined Redox Electrolytes and Porous Electrodes.Adv Mater. 2022 Aug;34(34):e2202380. doi: 10.1002/adma.202202380. Epub 2022 Jul 14. Adv Mater. 2022. PMID: 35413141 Review.
-
Redox deposition of nanoscale metal oxides on carbon for next-generation electrochemical capacitors.Acc Chem Res. 2013 May 21;46(5):1062-74. doi: 10.1021/ar2002717. Epub 2012 Mar 1. Acc Chem Res. 2013. PMID: 22380783
-
Ultrahigh volumetric capacitance and cyclic stability of fluorine and nitrogen co-doped carbon microspheres.Nat Commun. 2015 Sep 29;6:8503. doi: 10.1038/ncomms9503. Nat Commun. 2015. PMID: 26415838 Free PMC article.
-
A Review of Compact Carbon Design for Supercapacitors with High Volumetric Performance.Small. 2021 Dec;17(48):e2007548. doi: 10.1002/smll.202007548. Epub 2021 Mar 8. Small. 2021. PMID: 33682998 Review.
Cited by
-
Advances in Carbon Xerogels: Structural Optimization for Enhanced EDLC Performance.Gels. 2024 Jun 14;10(6):400. doi: 10.3390/gels10060400. Gels. 2024. PMID: 38920946 Free PMC article. Review.
-
One-pot assembling pyrroloquinoline quinone glucose dehydrogenase with polydopamine to overcome the reproducibility issues of layer-by-layer electrode development.Sens Diagn. 2025 Jun 20;4(9):750-8. doi: 10.1039/d5sd00053j. Online ahead of print. Sens Diagn. 2025. PMID: 40630738 Free PMC article.
References
-
- Tarascon J. M., Armand M., Nature 2001, 414, 359. - PubMed
-
- a) Pan Z., Liu X., Yang J., Li X., Liu Z., Loh X., Wang J., Adv. Energy Mater. 2021, 11, 2100608;
- b) Pan Z., Yang J., Jiang J., Qiu Y., Wang J., Mater. Today Energy 2020, 18, 100523;
- c) Zhong Y., Lu Y., Pan Z., Yang J., Du G., Chen J., Zhang Q., Zhou H., Wang J., Wang C., Li W., Adv. Funct. Mater. 2021, 31, 2009853;
- d) Qiu Y., Pan Z., Chen H., Ye D., Guo L., Fan Z., Yang S., Sci. Bull. 2019, 64, 1348; - PubMed
- e) Zeng Y., Zhang X., Qin R., Liu X., Fang P., Zheng D., Tong Y., Lu X., Adv. Mater. 2019, 31, 1903675. - PubMed
-
- Sun H., Mei L., Liang J., Zhao Z., Lee C., Fei H., Ding M., Lau J., Li M., Wang C., Xu X., Hao G., Papandrea B., Shakir I., Dunn B., Huang Y., Duan X., Science 2017, 356, 599. - PubMed
-
- a) Chen M., Pan Z., Jin X., Chen Z., Zhong Y., Wang X., Qiu Y., Xu M., Li W., Zhang Y., J. Mater. Chem. A 2019, 7, 4494;
- b) Zhong Y., Li B., Li S., Xu S., Pan Z., Huang Q., Xing L., Wang C., Li W., Nano‐Micro Lett. 2018, 10, 56; - PMC - PubMed
- c) Yang J., Liu M., Wei Z., Pan Z., Qiu Y., Ye F., Yang Y., Zhao X., Sheng L., Zhang Y., Electrochim. Acta 2016, 211, 671;
- d) Wang X., Pan Z., Yang J., Lyu Z., Zhong Y., Zhou G., Qiu Y., Zhang Y., Wang J., Li W., Energy Storage Mater. 2019, 22, 179;
- e) Dunn B., Kamath H., Tarascon J. M., Science 2011, 334, 928. - PubMed
-
- a) Simon P., Gogotsi Y., Dunn B., Science 2014, 343, 1210; - PubMed
- b) Yin J., Zhang W., Alhebshi N. A., Salah N., Alshareef H. N., Small Methods 2020, 4, 1900853;
- c) Lai F., Yang C., Lian R., Chu K., Qin J., Zong W., Rao D., Hofkens J., Lu X., Liu T., Adv. Mater. 2020, 32, 2002474; - PubMed
- d) Lv T., Liu M., Zhu D., Gan L., Chen T., Adv. Mater. 2018, 30, 1705489; - PubMed
- e) Luo H., Kaneti Y., Ai Y., Wu Y., Wei F., Fu J., Cheng J., Jing C., Yuliarto B., Eguchi M., Na J., Yamauchi Y., Liu S., Adv. Mater. 2021, 33, 2007318; - PubMed
- f) Yan Y., Chen G., She P., Zhong G., Yan W., Guan B., Yamauchi Y., Adv. Mater. 2020, 32, 2004654; - PubMed
- g) He Y., Zhuang X., Lei C., Lei L., Hou Y., Mai Y., Feng X., Nano Today 2019, 24, 103.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
