Synthesis and Reactivity of a Neutral Homocyclic Silylene
- PMID: 34797603
- PMCID: PMC9299817
- DOI: 10.1002/anie.202114485
Synthesis and Reactivity of a Neutral Homocyclic Silylene
Abstract
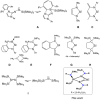
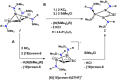
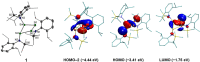
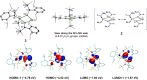
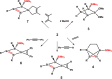
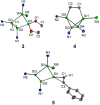
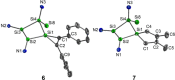
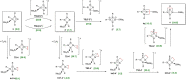
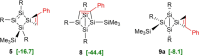
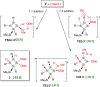
Isolation of the neutral homocyclic silylene 2 is possible via amine ligand abstraction with potassium graphite (KC8 ) and subsequent reaction with SiMe3 Cl from a bicyclic silicon(I) amide J. This reaction proceeds via an anionic homoaromatic silicon ring compound 1 as an intermediate. The twofold-coordinated silicon atom in the homocyclic silylene 2 is stabilized by an allyl-type π-electron delocalization. 2 reacts in an oxidative addition with two equivalents of MeOH and in cycloadditions with ethene, phenylacetylene, diphenylacetylene and with 2,3-dimethyl-1,3-butadiene to afford novel functionalized ring compounds.
Keywords: Homoaromaticity; Homocyclic; N ligands; Silylene; Small molecule activation.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- None
-
- Haaf M., Schmedake T., West R., Acc. Chem. Res. 2000, 33, 704–714; - PubMed
-
- Mizuhata Y., Sasamori T., Tokitoh N., Chem. Rev. 2009, 109, 3479–3511; - PubMed
-
- Asay M., Jones C., Driess M., Chem. Rev. 2011, 111, 354–396; - PubMed
-
- Gehrhus B., Lappert M. F., Heinicke J., Boese R., Bläser D., J. Chem. Soc. Chem. Commun. 1995, 1931–1932;
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

