COUPY Coumarins as Novel Mitochondria-Targeted Photodynamic Therapy Anticancer Agents
- PMID: 34797672
- PMCID: PMC8667040
- DOI: 10.1021/acs.jmedchem.1c01254
COUPY Coumarins as Novel Mitochondria-Targeted Photodynamic Therapy Anticancer Agents
Abstract
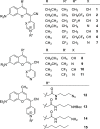
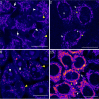
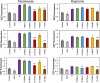
Photodynamic therapy (PDT) for cancer treatment has drawn increased attention over the last decades. Herein, we introduce a novel family of low-molecular-weight coumarins as potential PDT anticancer tools. Through a systematic study with a library of 15 compounds, we have established a detailed structure-activity relationship rationale, which allowed the selection of three lead compounds exhibiting effective in vitro anticancer activities upon visible-light irradiation in both normoxia and hypoxia (phototherapeutic indexes up to 71) and minimal toxicity toward normal cells. Acting as excellent theranostic agents targeting mitochondria, the mechanism of action of the photosensitizers has been investigated in detail in HeLa cells. The generation of cytotoxic reactive oxygen species, which has been found to be a major contributor of the coumarins' phototoxicity, and the induction of apoptosis and/or autophagy have been identified as the cell death modes triggered after irradiation with low doses of visible light.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

