Iota-carrageenan and xylitol inhibit SARS-CoV-2 in Vero cell culture
- PMID: 34797868
- PMCID: PMC8604354
- DOI: 10.1371/journal.pone.0259943
Iota-carrageenan and xylitol inhibit SARS-CoV-2 in Vero cell culture
Abstract
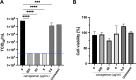
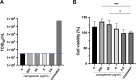
Last year observed a global pandemic caused by SARS-CoV-2 (severe acute respiratory syndrome-coronavirus 2) infection affecting millions of individuals worldwide. There is an urgent unmet need to provide an easily producible and affordable medicine to prevent transmission and provide early treatment for this disease. Since the nasal cavity and the rhinopharynx are the sites of initial replication of SARS-CoV-2, a nasal spray may be an effective option to target SARS-CoV-2 infection. In this study, we tested the antiviral action of three candidate nasal spray formulations against SARS-CoV-2 in vitro. We determined that iota-carrageenan in concentrations as low as 6 μg/mL inhibits SARS-CoV-2 in vitro. The concentrations of iota-carrageenan with activity against SARS-CoV-2 in vitro may be easily achieved through the application of nasal sprays as commonly used in several countries. Recently a double-blind, placebo-controlled study showed that iota-carrageenan in isotonic sodium chloride reduces ca. five times the risk of infection by SARS-CoV-2 in health care personnel. Further, xylitol at a concentration of 50 mg/mL (ca. 329 mM) was found to exert some antiviral action, though this preliminary finding needs further confirmation.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: I have a commercial affiliation to Laboratorio Pablo Cassará S.R.L, who provided the formulations for the study and to Amcyte Pharma Inc.. This submission is related to US Patent No. 11.013.687 “PREVENTIVE AND THERAPEUTIC TREATMENT FOR COVID 19 AND ANY OTHER DISEASE CAUSED BY SARS COV 2”, whose inventor is Julio César Vega. This does not alter our adherence to PLOS ONE’s policies on sharing data and materials.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

