An Analysis of Metabolic Changes in the Retina and Retinal Pigment Epithelium of Aging Mice
- PMID: 34797906
- PMCID: PMC8606884
- DOI: 10.1167/iovs.62.14.20
An Analysis of Metabolic Changes in the Retina and Retinal Pigment Epithelium of Aging Mice
Abstract
Purpose: The purpose of this study was to present our hypothesis that aging alters metabolic function in ocular tissues. We tested the hypothesis by measuring metabolism in aged murine tissues alongside retinal responses to light.
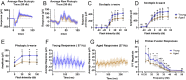
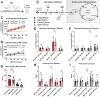
Methods: Scotopic and photopic electroretinogram (ERG) responses in young (3-6 months) and aged (23-26 months) C57Bl/6J mice were recorded. Metabolic flux in retina and eyecup explants was quantified using U-13C-glucose or U-13C-glutamine with gas chromatography-mass spectrometry (GC-MS), O2 consumption rate (OCR) in a perifusion apparatus, and quantifying adenosine triphosphatase (ATP) with a bioluminescence assay.
Results: Scotopic and photopic ERG responses were reduced in aged mice. Glucose metabolism, glutamine metabolism, OCR, and ATP pools in retinal explants were mostly unaffected in aged mice. In eyecups, glutamine usage in the Krebs Cycle decreased while glucose metabolism, OCR, and ATP pools remained stable.
Conclusions: Our examination of metabolism showed negligible impact of age on retina and an impairment of glutamine anaplerosis in eyecups. The metabolic stability of these tissues ex vivo suggests age-related metabolic alterations may not be intrinsic. Future experiments should focus on determining whether external factors including nutrient supply, oxygen availability, or structural changes influence ocular metabolism in vivo.
Conflict of interest statement
Disclosure:
Figures


References
-
- Elliott DB, Yang KCH, Whitaker D.. Visual acuity changes throughout adulthood in normal, healthy eyes: Seeing beyond 6/6. Optom Vis Sci. 1995; 72(3): 186–191. - PubMed
-
- Ferrer-Blasco T, González-Méijome JM, Montés-Micó R.. Age-related changes in the human visual system and prevalence of refractive conditions in patients attending an eye clinic. J Cataract Refract Surg. 2008; 34(3): 424–432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

