Mosaic human preimplantation embryos and their developmental potential in a prospective, non-selection clinical trial
- PMID: 34798051
- PMCID: PMC8715143
- DOI: 10.1016/j.ajhg.2021.11.002
Mosaic human preimplantation embryos and their developmental potential in a prospective, non-selection clinical trial
Abstract
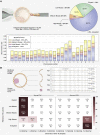
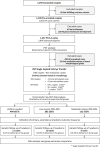
Chromosome imbalance (aneuploidy) is the major cause of pregnancy loss and congenital disorders in humans. Analyses of small biopsies from human embryos suggest that aneuploidy commonly originates during early divisions, resulting in mosaicism. However, the developmental potential of mosaic embryos remains unclear. We followed the distribution of aneuploid chromosomes across 73 unselected preimplantation embryos and 365 biopsies, sampled from four multifocal trophectoderm (TE) samples and the inner cell mass (ICM). When mosaicism impacted fewer than 50% of cells in one TE biopsy (low-medium mosaicism), only 1% of aneuploidies affected other portions of the embryo. A double-blinded prospective non-selection trial (NCT03673592) showed equivalent live-birth rates and miscarriage rates across 484 euploid, 282 low-grade mosaic, and 131 medium-grade mosaic embryos. No instances of mosaicism or uniparental disomy were detected in the ensuing pregnancies or newborns, and obstetrical and neonatal outcomes were similar between the study groups. Thus, low-medium mosaicism in the trophectoderm mostly arises after TE and ICM differentiation, and such embryos have equivalent developmental potential as fully euploid ones.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.C., M.P., L.G., C.P., M.F., M.F., J.C., and C.R. are full-time employees of Igenomix. L.R. is the Scientific Director of GeneraLife IVF. She is editor of reproductive biomedicine online and has been associate editor of human reproduction update. She has been the principal investigator of a study sponsored by Merck KGaA. She has received honoraria and consultation fees from Merck, MSD, Ferring, Ibsa, Cooper Surgical, Cook, Nterilizer, Fujifilm-Irvine Scientific, Medea, and Universal Clinics. She is a partner and shareholder of Global Investment Clinics, Genera Health Care, and Nterilizer and has been affiliated with Flam. D.C. is a full-time employee of GeneraLife IVF, where he is the science and research manager. He received paid lectures from Fujifilm-Irvine Scientific. He received paid consultations from Merck. F.B. reports personal fees from Fujifilm-Irvine Scientific, outside the submitted work. A.V. reports personal fees from Gedeon Richter, personal fees from Merck, and personal fees from MSD, outside the submitted work. E.H. and I.V. are supported by an ERC consolidator grant (724718-ReCAP), the Novo Nordisk Foundation Young Investigator Award (NNF15OC0016662), ReproUnion, and the Danish National Research Foundation (center grant 6110-00344B). The views expressed in this article are those of the authors and not necessarily those of the sponsors. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of this article. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. E.H. is the executive board member and chair of the scientific advisory board of ReproUnion, which is co-funded by Ferring Pharmaceuticals. F.M.U. is the scientific director of GeneraLife IVF. He is the president of the Italian Society of Fertility and Sterility (SIFES). He has been the principal investigator of a study sponsored by Merck and by S&R Farmaceutici. He has received honoraria and consultation fees from Merck, Merck Sharp and Dohnme Corporation, Ferring, Institut Biochimique, Cooper Surgical, Cook, Nterilizer, Fujifilm-Irvine Scientific, Medea, and Universal Clinics. He is partner/shareholder of Global Investment Clinics, Genera Health Care, and Nterilizer, and has been of Flam. C.S. is the head of the scientific advisory board of Igenomix. The other authors declare no competing interests.
Figures

References
-
- Popovic M., Dhaenens L., Boel A., Menten B., Heindryckx B. Chromosomal mosaicism in human blastocysts: the ultimate diagnostic dilemma. Hum. Reprod. Update. 2020;26:313–334. - PubMed
-
- van Echten-Arends J., Mastenbroek S., Sikkema-Raddatz B., Korevaar J.C., Heineman M.J., van der Veen F., Repping S. Chromosomal mosaicism in human preimplantation embryos: a systematic review. Hum. Reprod. Update. 2011;17:620–627. - PubMed
-
- van der Meij K.R.M., Sistermans E.A., Macville M.V.E., Stevens S.J.C., Bax C.J., Bekker M.N., Bilardo C.M., Boon E.M.J., Boter M., Diderich K.E.M., et al. Dutch NIPT Consortium TRIDENT-2: National Implementation of Genome-wide Non-invasive Prenatal Testing as a First-Tier Screening Test in the Netherlands. Am. J. Hum. Genet. 2019;105:1091–1101. - PMC - PubMed
-
- Van Den Bogaert K., Lannoo L., Brison N., Gatinois V., Baetens M., Blaumeiser B., Boemer F., Bourlard L., Bours V., De Leener A., et al. Outcome of publicly funded nationwide first-tier noninvasive prenatal screening. Genet. Med. 2021;23:1137–1142. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

