Efficacy of Severe Acute Respiratory Syndrome Coronavirus-2 Vaccine in Patients With Thoracic Cancer: A Prospective Study Supporting a Third Dose in Patients With Minimal Serologic Response After Two Vaccine Doses
- PMID: 34798306
- PMCID: PMC8593625
- DOI: 10.1016/j.jtho.2021.10.015
Efficacy of Severe Acute Respiratory Syndrome Coronavirus-2 Vaccine in Patients With Thoracic Cancer: A Prospective Study Supporting a Third Dose in Patients With Minimal Serologic Response After Two Vaccine Doses
Abstract
Introduction: Coronavirus disease 2019 resulted in a 30% mortality rate in patients with thoracic cancer. Given that patients with cancer were excluded from serum antisevere acute respiratory syndrome coronavirus-2 (SARS-CoV-2) vaccine registration trials, it is still unknown whether they would develop a protective antispike antibody response after vaccination. This prospective vaccine monitoring study primarily aimed to assess humoral responses to the SARS-CoV-2 vaccine in patients with thoracic cancer.
Methods: SARS-CoV-2-spike antibodies were measured using the Abbot Architect SARS-CoV-2 immunoglobulin G immunoassay before the first injection of BNT162b2 mRNA vaccine, at week 4, and 2 to 16 weeks after the second vaccine dose administration. The factors associated with antibody response were analyzed.
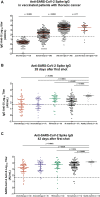
Results: Overall, 306 patients, with a median age of 67.0 years (interquartile range: 58-74), were vaccinated. Of these, 283 patients received two vaccine doses at 28-day intervals. After a 6.7-month median follow-up, eight patients (2.6%) contracted proven symptomatic SARS-CoV-2 infection, with rapid favorable evolution. Of the 269 serologic results available beyond day 14 after the second vaccine dose administration, 17 patients (6.3%) were still negative (<50 arbitrary units/mL, whereas 34 (11%) were less than 300 arbitrary units/mL (12.5th percentile). In multivariate analysis, only age (p < 0.01) and long-term corticosteroid treatment (p = 0.01) were significantly associated with a lack of immunization. A total of 30 patients received a third vaccine dose, with only three patients showing persistently negative serology thereafter, whereas the others exhibited clear seroconversion.
Conclusions: SARS-CoV2 vaccines were found to be efficient in patients with thoracic cancer, most of them being immunized after two doses. A third shot given to 1% of patients with persistent low antibody titers resulted in an 88% immunization rate.
Keywords: Antibody response; COVID-19; Lung cancer; SARS-CoV-2; mRNA vaccine.
Copyright © 2021 International Association for the Study of Lung Cancer. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Lièvre A., Turpin A., Ray-Coquard I., et al. Risk factors for coronavirus disease 2019 (COVID-19) severity and mortality among solid patients with cancer and impact of the disease on anticancer treatment: a French nationwide cohort study (GCO-002 CACOVID-19) Eur J Cancer. 2020;141:62–81. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

