MSMEG_3955 from Mycobacterium smegmatis is a FMN bounded homotrimeric NAD(P)H:Flavin mononucleotide (FMN) oxidoreductase
- PMID: 34798816
- PMCID: PMC8605562
- DOI: 10.1186/s12866-021-02330-y
MSMEG_3955 from Mycobacterium smegmatis is a FMN bounded homotrimeric NAD(P)H:Flavin mononucleotide (FMN) oxidoreductase
Abstract
Background: Tuberculosis (TB) remains an important public health problem since it is the major cause of elevated morbidity and mortality globally. Previous works have shown that Mycobacterium tuberculosis (Mtb); the prime causative agent of the deadly disease has dormancy survival regulator (DosR) regulon, a two-component regulatory system which controls the transcription of more than 50 genes. However, the structure and detailed functions of these DosR regulated genes are largely undetermined. Out of many DosR regulon genes, Rv3131 gets up regulated in hypoxic conditions and was believed to encode for a nitroreductase flavoprotein. The utilization of mycobacteria-specific model systems has greatly added to our understanding of the molecular mechanisms involved in the life cycle and pathogenesis of Mtb.
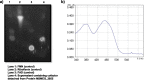
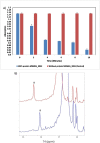
Results: In this study the non-pathogenic mycobacterial model organism Mycobacterium smegmatis (Msmeg) was used to reveal the structure and function of MSMEG_3955; which is a homologue of Rv3131 from Mtb. Using chromatography and spectroscopy techniques it was revealed that cofactor flavin mononucleotide (FMN) was bound to flavoprotein MSMEG_3955. Consistent with the homology modelling predictions, Circular Dichroism (CD) analysis indicated that the MSMEG_3955 is composed of 39.3% α-helix and 24.9% β-pleated sheets. In contrast to the current notions, the enzymatic assays performed in the present study revealed that MSMEG_3955 was not capable of reducing nitro substrates but showed NADPH dependent FMN oxidoreductase activity. Also, gel permeation chromatography, dynamic light scattering and native acidic gels showed that MSMEG_3955 exists as a homotrimer. Furthermore, the presence of NADPH dependent FMN oxidoreductase and homotrimeric existence could be an alternative function of the protein to help the bacteria survive in dormant state or may be involved in other biochemical pathways.
Conclusion: MSMEG_3955 is a FMN bound flavoprotein, which exits as a trimer under in vitro conditions. There is no disulphide linkages in between the three protomers of the homotrimer MSMEG_3955. It has a NADPH dependent FMN oxidoreductase activity.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Purkayastha A, McCue LA, McDonough KA. Identification of a mycobacterium tuberculosis putative classical nitroreductase gene whose expression is coregulated with that of the acr gene within macrophages, in standing versus shaking cultures, and under low oxygen conditions. Infect Immun. 2002;70:1518–1529. doi: 10.1128/IAI.70.3.1518-1529.2002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

