Xanthine Oxidoreductase Inhibitors Suppress the Onset of Exercise-Induced AKI in High HPRT Activity Urat1- Uox Double Knockout Mice
- PMID: 34799437
- PMCID: PMC8819989
- DOI: 10.1681/ASN.2021050616
Xanthine Oxidoreductase Inhibitors Suppress the Onset of Exercise-Induced AKI in High HPRT Activity Urat1- Uox Double Knockout Mice
Abstract
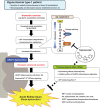
Background: Hereditary renal hypouricemia type 1 (RHUC1) is caused by URAT1/SLC22A12 dysfunction, resulting in urolithiasis and exercise-induced AKI (EIAKI). However, because there is no useful experimental RHUC1 animal model, the precise pathophysiologic mechanisms underlying EIAKI have yet to be elucidated. We established a high HPRT activity Urat1-Uox double knockout (DKO) mouse as a novel RHUC1 animal model for investigating the cause of EIAKI and the potential therapeutic effect of xanthine oxidoreductase inhibitors (XOIs).
Methods: The novel Urat1-Uox DKO mice were used in a forced swimming test as loading exercise to explore the onset mechanism of EIAKI and evaluate related purine metabolism and renal injury parameters.
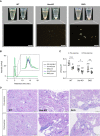
Results: Urat1-Uox DKO mice had uricosuric effects and elevated levels of plasma creatinine and BUN as renal injury markers, and decreased creatinine clearance observed in a forced swimming test. In addition, Urat1-Uox DKO mice had increased NLRP3 inflammasome activity and downregulated levels of Na+-K+-ATPase protein in the kidney, as Western blot analysis showed. Finally, we demonstrated that topiroxostat and allopurinol, XOIs, improved renal injury and functional parameters of EIAKI.
Conclusions: Urat1-Uox DKO mice are a useful experimental animal model for human RHUC1. The pathogenic mechanism of EIAKI was found to be due to increased levels of IL-1β via NLRP3 inflammasome signaling and Na+-K+-ATPase dysfunction associated with excessive urinary urate excretion. In addition, XOIs appear to be a promising therapeutic agent for the treatment of EIAKI.
Keywords: exercise-induced acute kidney injury (EIAKI); hypoxanthine phosphoribosyltransferase (HPRT); inborn errors; knockout; mice; renal hypouricemia (RHUC); renal tubular transport; urate transporter 1 (URAT1); urolithiasis; xanthine oxidoreductase inhibitor (XOI).
Copyright © 2022 by the American Society of Nephrology.
Figures






References
-
- Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, et al. : Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 417: 447–452, 2002 - PubMed
-
- Ichida K, Hosoyamada M, Hisatome I, Enomoto A, Hikita M, Endou H, et al. : Clinical and molecular analysis of patients with renal hypouricemia in Japan-influence of URAT1 gene on urinary urate excretion. J Am Soc Nephrol 15: 164–173, 2004 - PubMed
-
- Ichida K, Hosoyamada M, Kamatani N, Kamitsuji S, Hisatome I, Shibasaki T, et al. : Age and origin of the G774A mutation in SLC22A12 causing renal hypouricemia in Japanese. Clin Genet 74: 243–251, 2008 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

