Deficient neurotransmitter systems and synaptic function in frontotemporal lobar degeneration-Insights into disease mechanisms and current therapeutic approaches
- PMID: 34799692
- PMCID: PMC9095474
- DOI: 10.1038/s41380-021-01384-8
Deficient neurotransmitter systems and synaptic function in frontotemporal lobar degeneration-Insights into disease mechanisms and current therapeutic approaches
Abstract
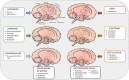
Frontotemporal lobar degeneration (FTLD) comprises a heterogenous group of fatal neurodegenerative diseases and, to date, no validated diagnostic or prognostic biomarkers or effective disease-modifying therapies exist for the different clinical or genetic subtypes of FTLD. Current treatment strategies rely on the off-label use of medications for symptomatic treatment. Changes in several neurotransmitter systems including the glutamatergic, GABAergic, dopaminergic, and serotonergic systems have been reported in FTLD spectrum disease patients. Many FTLD-related clinical and neuropsychiatric symptoms such as aggressive and compulsive behaviour, agitation, as well as altered eating habits and hyperorality can be explained by disturbances in these neurotransmitter systems, suggesting that their targeting might possibly offer new therapeutic options for treating patients with FTLD. This review summarizes the present knowledge on neurotransmitter system deficits and synaptic dysfunction in model systems and patients harbouring the most common genetic causes of FTLD, the hexanucleotide repeat expansion in C9orf72 and mutations in the granulin (GRN) and microtubule-associated protein tau (MAPT) genes. We also describe the current pharmacological treatment options for FLTD that target different neurotransmitter systems.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

