Improved survival in patients with unresectable stage III EGFR-mutant adenocarcinoma with upfront EGFR-tyrosine kinase inhibitors
- PMID: 34799993
- PMCID: PMC8758433
- DOI: 10.1111/1759-7714.14237
Improved survival in patients with unresectable stage III EGFR-mutant adenocarcinoma with upfront EGFR-tyrosine kinase inhibitors
Abstract
Background: Although epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) have been the standard treatment for advanced EGFR-mutant adenocarcinoma, the effects of upfront EGFR-TKI use in unresectable stage III EGFR-mutant adenocarcinoma remain unexplored. Here, we conducted a retrospective study to compare different treatment strategies in these patients.
Methods: From October 2010 to June 2019, patients with unresectable stage III adenocarcinoma who received treatment at a tertiary referral center were enrolled. Patients were classified into three groups: EGFR-mutant adenocarcinoma treated with concurrent chemoradiotherapy (group 1) or EGFR-TKI (group 2) and EGFR wild-type adenocarcinoma treated with concurrent chemoradiotherapy (group 3). Progression-free survival, progression-free survival-2, and overall survival were estimated and compared using Kaplan-Meier and log-rank tests.
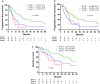
Results: A total of 92 patients were enrolled; 10, 40, and 42 patients were assigned to groups 1, 2, and 3, respectively. Patients with EGFR mutations who received upfront EGFR-TKIs had significantly longer progression-free and overall survival than those who received upfront concurrent chemoradiotherapy (hazard ratio 0.33 vs. 0.34, p = 0.006 vs. 0.031) according to a Cox model adjusted for possible confounders. Moreover, upfront concurrent chemoradiotherapy did not lead to higher survival rates in patients with EGFR mutations than in those with EGFR wild-type adenocarcinoma (progression-free survival; hazard ratio 0.37, p = 0.036; overall survival; hazard ratio 0.35, p = 0.080) by Cox regression analysis.
Conclusion: This current study suggests that EGFR-TKIs is a better choice for patients with unresectable stage III EGFR-mutant adenocarcinoma. However, further randomized studies are required to validate the results.
Keywords: adenocarcinoma; chemoradiotherapy; epidermal growth factor receptor; stage III; tyrosine kinase inhibitors.
© 2021 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Wang BY, Huang JY, Cheng CY, Lin CH, Ko J, Liaw YP. Lung cancer and prognosis in Taiwan: a population‐based cancer registry. J Thorac Oncol. 2013;8:1128–35. - PubMed
-
- Aupérin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta‐analysis of concomitant versus sequential radiochemotherapy in locally advanced non‐small‐cell lung cancer. J Clin Oncol. 2010;28:2181–90. - PubMed
-
- Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib versus cisplatin‐based chemotherapy for EGFR mutation‐positive lung adenocarcinoma (LUX‐Lung 3 and LUX‐Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

