Pharmacological blockage of the AHR-CYP1A1 axis: a call for in vivo evidence
- PMID: 34800164
- PMCID: PMC8605459
- DOI: 10.1007/s00109-021-02163-2
Pharmacological blockage of the AHR-CYP1A1 axis: a call for in vivo evidence
Abstract
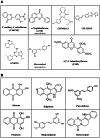
The aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor that can be activated by structurally diverse compounds arising from the environment and the microbiota and host metabolism. Expanding evidence has been shown that the modulation of the canonical pathway of AHR occurs during several chronic diseases and that its abrogation might be of clinical interest for metabolic and inflammatory pathological processes. However, most of the evidence on the pharmacological abrogation of the AHR-CYP1A1 axis has been reported in vitro, and therefore, guidance for in vivo studies is needed. In this review, we cover the state-of-the-art of the pharmacodynamic and pharmacokinetic properties of AHR antagonists and CYP1A1 inhibitors in different in vivo rodent (mouse or rat) models of disease. This review will serve as a road map for those researchers embracing this emerging therapeutic area targeting the AHR. Moreover, it is a timely opportunity as the first AHR antagonists have recently entered the clinical stage of drug development.
Keywords: Aryl hydrocarbon receptor; CYP1A1; In vivo; Inflammation; Metabolism; Oxidative stress.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Degroot D, He G, Fraccalvieri D, Bonati L, Pandini AA, Denison MS (2011) AHR Ligands: promiscuity in Binding and Diversity in Response, in: AH Recept. Biol. Toxicol., John Wiley and Sons, Hoboken, NJ, USA, pp. 63–79. 10.1002/9781118140574.ch4
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

