Burden of disease in myasthenia gravis: taking the patient's perspective
- PMID: 34800167
- PMCID: PMC9120127
- DOI: 10.1007/s00415-021-10891-1
Burden of disease in myasthenia gravis: taking the patient's perspective
Erratum in
-
Correction to: Burden of disease in myasthenia gravis: taking the patient's perspective.J Neurol. 2022 Oct;269(10):5688-5689. doi: 10.1007/s00415-022-11290-w. J Neurol. 2022. PMID: 35960393 Free PMC article. No abstract available.
Abstract
Background: Myasthenia gravis (MG) leads to exertion-dependent muscle weakness, but also psychological and social well-being are limited. We aim to describe the burden of disease in MG including sociodemographic, economical, psychosocial as well as clinical aspects, to compare health-related quality of life (HRQoL) of patients with MG to the general population (genP) and to explore risk factors for a lower HRQoL.
Methods: This case-control study was conducted with MG patients of the German Myasthenia Association. A questionnaire-based survey included sociodemographic and clinical data as well as standardized questionnaires, e.g. the Short Form Health (SF-36). HRQoL was compared to genP in a matched-pairs analysis. Participants of the German Health Interview and Examination Survey for Adults (DEGS1) served as control group.
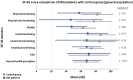
Results: In our study, 1660 MG patients participated and were compared to 2556 controls from the genP. Patients with MG showed lower levels of physical functioning (SF-36 mean 56.0, SD 30.3) compared to the genP (mean 81.8, SD 22.1, adjusted difference: 25, 95% CI 22-29) and lower mental health sub-score (SF-36 mean 67.3, SD 19.8, vs. 74.1, SD 16.7, adjusted difference: 5, 95% CI 2-8). Female gender, higher age, low income, partnership status, lower activities of daily life, symptoms of depression, anxiety and fatigue and self-perceived low social support were associated with a lower HRQoL in MG patients.
Discussion: HRQoL is lower in patients with MG compared to genP. The burden of MG on patients includes economic and social aspects as well as their emotional well-being. New therapies must achieve improvements for patients in these areas.
Trial registration information: Clinicaltrials.gov, NCT03979521, submitted: June 7, 2019, first patient enrolled: May 1, 2019, https://clinicaltrials.gov/ct2/show/NCT03979521.
Keywords: Burden of disease; Myasthenia gravis; Quality of life; Real-world setting.
© 2021. The Author(s).
Conflict of interest statement
Financial: Sophie Lehnerer and Sarah Hoffmann have received speaker’s honoraria and honoraria for attendance at advisory boards from Alexion. Maike Krause received speaker’s honoraria from Argnx. Frauke Stascheit received speaker’s honoraria from Alexion. Andreas Meisel received speaker’s honoraria from Alexion, Grifols and Hormosan. He received honoraria from Alexion, UCB, MorphoSys and Argnx for consulting services and financial research support from Octapharma and Alexion. All other authors report no disclosures relevant to the manuscript. Non-financial: Andreas Meisel is Chairman of the medical advisory board of the German Myasthenia Gravis Society.
Figures

References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

