Safety, immunogenicity, and efficacy of a COVID-19 vaccine (NVX-CoV2373) co-administered with seasonal influenza vaccines: an exploratory substudy of a randomised, observer-blinded, placebo-controlled, phase 3 trial
- PMID: 34800364
- PMCID: PMC8598212
- DOI: 10.1016/S2213-2600(21)00409-4
Safety, immunogenicity, and efficacy of a COVID-19 vaccine (NVX-CoV2373) co-administered with seasonal influenza vaccines: an exploratory substudy of a randomised, observer-blinded, placebo-controlled, phase 3 trial
Abstract
Background: The safety and immunogenicity profile of COVID-19 vaccines when administered concomitantly with seasonal influenza vaccines have not yet been reported. We therefore aimed to report the results of a substudy within a phase 3 UK trial, by evaluating the safety, immunogenicity, and efficacy of NVX-CoV2373 when co-administered with licensed seasonal influenza vaccines.
Methods: We did a planned exploratory substudy as part of the randomised, observer-blinded, placebo-controlled, phase 3 trial of the safety and efficacy of the COVID-19 vaccine (NVX-CoV2373) by co-administrating the influenza vaccine at four study hospitals in the UK. Approximately, the first 400 participants meeting the main study entry criteria-with no contraindications to influenza vaccination-were invited to join the substudy. Participants of the main study were randomly assigned (1:1) to receive two intramuscular injections of either NVX-CoV2373 (5 μg) or placebo (normal saline) 21 days apart; participants enrolled into the substudy were co-vaccinated with a single (0·5 mL) intramuscular, age-appropriate (quadrivalent influenza cell-based vaccine [Flucelvax Quadrivalent; Seqirus UK, Maidenhead] for those aged 18-64 years and adjuvanted trivalent influenza vaccine [Fluad; Seqirus UK, Maidenhead] for those ≥65 years), licensed, influenza vaccine on the opposite deltoid to that of the first study vaccine dose or placebo. The influenza vaccine was administered in an open-label manner and at the same time as the first study injection. Reactogenicity was evaluated via an electronic diary for 7 days after vaccination in addition to monitoring for unsolicited adverse events, medically attended adverse events, and serious adverse events. Immunogenicity was assessed with influenza haemagglutination inhibition and SARS-CoV-2 anti-spike protein IgG assays. Vaccine efficacy against PCR-confirmed, symptomatic COVID-19 was assessed in participants who were seronegative at baseline, received both doses of study vaccine or placebo, had no major protocol deviations affecting the primary endpoint, and had no confirmed cases of symptomatic COVID-19 from the first dose until 6 days after the second dose (per-protocol efficacy population). Immunogenicity was assessed in participants who received scheduled two doses of study vaccine, had a baseline sample and at least one post-vaccination sample, and had no major protocol violations before unmasking (per-protocol immunogenicity population). Reactogenicity was analysed in all participants who received at least one dose of NVX-CoV2373 or placebo and had data collected for reactogenicity events. Safety was analysed in all participants who received at least one dose of NVX-CoV2373 or placebo. Comparisons were made between participants of the substudy and the main study (who were not co-vaccinated for influenza). This study is registered with ClinicalTrials.gov, number NCT04583995.
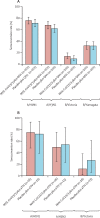
Findings: Between Sept 28, 2020, and Nov 28, 2020, a total of 15 187 participants were randomised into the main phase 3 trial, of whom 15 139 received treatment (7569 received dose one of NVX-CoV2373 and 7570 received dose one of placebo). 431 participants were co-vaccinated with a seasonal influenza vaccine in the substudy (217 received NVX-CoV2373 plus the influenza vaccine and 214 received placebo plus the influenza vaccine). In general, the substudy participants were younger, more racially diverse, and had fewer comorbid conditions than those in the main study. Reactogenicity events were more common in the co-administration group than in the NVX-CoV2373 alone group: tenderness (113 [64·9%] of 174 vs 592 [53·3%] of 1111) or pain (69 [39·7%] vs 325 [29·3%]) at injection site, fatigue (48 [27·7%] vs 215 [19·4%]), and muscle pain (49 [28·3%] vs 237 [21·4%]). Incidences of unsolicited adverse events, treatment-related medically attended adverse events, and serious adverse events were low and balanced between the co-administration group and the NVX-CoV2373 alone group. No episodes of anaphylaxis or deaths were reported within the substudy. Co-administration resulted in no change to influenza vaccine immune response although a reduction in antibody responses to the NVX-CoV2373 vaccine was noted. NVX-CoV2373 vaccine efficacy in the substudy (ie, participants aged 18 to <65 years) was 87·5% (95% CI -0·2 to 98·4) and in the main study was 89·8% (95% CI 79·7-95·5).
Interpretation: To our knowledge, this substudy is the first to show the safety, immunogenicity, and efficacy profile of a COVID-19 vaccine when co-administered with seasonal influenza vaccines. Our results suggest concomitant vaccination might be a viable immunisation strategy.
Funding: Novavax.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests ST, JSP, LK, FD, GG, IC, AR, and EJR are Novavax employees; and SR, JE, and AG-J are Seqirus employees, as they receive a salary for their work. All other authors declare no competing interests.
Figures


References
-
- WHO . World Health Organization; Geneva: 2019. WHO coronavirus (COVID-19) dashboard.https://covid19.who.int/
-
- Public Health England Annual flu programme. 2013. https://www.gov.uk/government/collections/annual-flu-programme
-
- US Centers for Disease Control and Prevention Interim clinical considerations for use of COVID-19 vaccines currently authorized in the United States. 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considera...
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

