FBXO7 triggers caspase 8-mediated proteolysis of the transcription factor FOXO4 and exacerbates neuronal cytotoxicity
- PMID: 34800438
- PMCID: PMC8665361
- DOI: 10.1016/j.jbc.2021.101426
FBXO7 triggers caspase 8-mediated proteolysis of the transcription factor FOXO4 and exacerbates neuronal cytotoxicity
Abstract
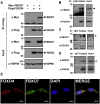
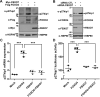
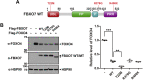
Parkinson's disease (PD) is characterized by the progressive loss of midbrain dopamine neurons in the substantia nigra. Mutations in the F-box only protein 7 gene (Fbxo7) have been reported to cause an autosomal recessive form of early-onset familial PD. FBXO7 is a part of the SKP1-Cullin1-F-box (SCF) E3 ubiquitin ligase complex, which mediates ubiquitination of numerous substrates. FBXO7 also regulates mitophagy, cell growth, and proteasome activity. A member of the FOXO family, the transcription factor FOXO4, is also known to modulate several cellular responses, including cell cycle progression and apoptosis; however, the relationship between FBXO7 and FOXO4 has not been investigated. In this study, we determined that FBXO7 binds to FOXO4 and negatively regulates intracellular FOXO4 levels. Interestingly, we also found that FBXO7-mediated degradation of FOXO4 did not occur through either of two major proteolysis systems, the ubiquitin-proteasome system or the lysosome-autophagy pathway, although it was blocked by a caspase 8-specific inhibitor and caspase 8-knockdown. Moreover, intracellular FOXO4 levels were greatly reduced in dopaminergic MN9D cells following treatment with neurotoxic 6-hydroxydopamine (6-OHDA), which was produced upon FBXO7-mediated and caspase 8-mediated proteolysis. Taken together, these results suggest that FOXO4 is negatively regulated in FBXO7-linked PD through caspase 8 activation, suppressing the cytoprotective effect of FOXO4 during 6-OHDA-induced neuronal cell death.
Keywords: 6-OHDA; FBXO7; FOXO4; Parkinson’s disease; caspase 8; neuronal cell death.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflict of interest with the content of this article.
Figures





References
-
- de Lau L.M.L., Breteler M.M.B. Epidemiology of Parkinson's disease. Lancet Neurol. 2016;5:525–535. - PubMed
-
- Wakabayashi K., Tanji K., Mori F., Takahashi H. The Lewy body in Parkinson’s disease: Molecules implicated in the formation and degradation of α-synuclein aggregates. Neuropathology. 2007;27:494–506. - PubMed
-
- Antony P.M., Diederich N.J., Kruger R., Balling R. The hallmarks of Parkinson’s disease. FEBS J. 2013;280:5981–5993. - PubMed
-
- Zhou Z.D., Lee J.C.T., Tan E.K. Pathophysiological mechanisms linking F-box only protein 7 (FBXO7) and Parkinson's disease (PD) Mutat. Res. 2018;778:72–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

