Safety and efficacy of a three-dose regimen of Plasmodium falciparum sporozoite vaccine in adults during an intense malaria transmission season in Mali: a randomised, controlled phase 1 trial
- PMID: 34801112
- PMCID: PMC8981424
- DOI: 10.1016/S1473-3099(21)00332-7
Safety and efficacy of a three-dose regimen of Plasmodium falciparum sporozoite vaccine in adults during an intense malaria transmission season in Mali: a randomised, controlled phase 1 trial
Abstract
Background: WHO recently approved a partially effective vaccine that reduces clinical malaria in children, but increased vaccine activity is required to pursue malaria elimination. A phase 1 clinical trial was done in Mali, west Africa, to assess the safety, immunogenicity, and protective efficacy of a three-dose regimen of Plasmodium falciparum sporozoite (PfSPZ) Vaccine (a metabolically active, non-replicating, whole malaria sporozoite vaccine) against homologous controlled human malaria infection (CHMI) and natural P falciparum infection.
Methods: We recruited healthy non-pregnant adults aged 18-50 years in Donéguébougou, Mali, and surrounding villages (Banambani, Toubana, Torodo, Sirababougou, Zorokoro) for an open-label, dose-escalation pilot study and, thereafter, a randomised, double-blind, placebo-controlled main trial. Pilot study participants were enrolled on an as-available basis to one group of CHMI infectivity controls and three staggered vaccine groups receiving: one dose of 4·5 × 105, one dose of 9 × 105, or three doses of 1·8 × 106 PfSPZ via direct venous inoculation at approximately 8 week intervals, followed by homologous CHMI 5 weeks later with infectious PfSPZ by direct venous inoculation (PfSPZ Challenge). Main cohort participants were stratified by village and randomly assigned (1:1) to receive three doses of 1·8 × 106 PfSPZ or normal saline at 1, 13, and 19 week intervals using permuted block design by the study statistician. The primary outcome was safety and tolerability of at least one vaccine dose; the secondary outcome was vaccine efficacy against homologous PfSPZ CHMI (pilot study) or against naturally transmitted P falciparum infection (main study) measured by thick blood smear. Combined artesunate and amodiaquine was administered to eliminate pre-existing parasitaemia. Outcomes were analysed by modified intention to treat (mITT; including all participants who received at least one dose of investigational product; safety and vaccine efficacy) and per protocol (vaccine efficacy). This trial is registered with ClinicalTrials.gov, number NCT02627456.
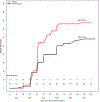
Findings: Between Dec 20, 2015, and April 30, 2016, we enrolled 56 participants into the pilot study (five received the 4·5 × 105 dose, five received 9 × 105, 30 received 1·8 × 106, 15 were CHMI controls, and one withdrew before vaccination) and 120 participants into the main study cohort with 60 participants assigned PfSPZ Vaccine and 60 placebo in the main study. Adverse events and laboratory abnormalities post-vaccination in all dosing groups were few, mainly mild, and did not differ significantly between vaccine groups (all p>0·05). Unexpected severe transaminitis occured in four participants: one participant in pilot phase that received 1·8 × 106 PfSPZ Vaccine, one participant in main phase that received 1·8 × 106 PfSPZ Vaccine, and two participants in the main phase placebo group. During PfSPZ CHMI, approximately 5 weeks after the third dose of 1·8 × 106 PfSPZ, none of 29 vaccinees and one of 15 controls became positive on thick blood smear; subsequent post-hoc PCR analysis for submicroscopic blood stage infections detected P falciparum parasites in none of the 29 vaccine recipients and eight of 15 controls during CHMI. In the main trial, 32 (58%) of 55 vaccine recipients and 42 (78%) of 54 controls became positive on thick blood smear during 24-week surveillance after vaccination. Vaccine efficacy (1-hazard ratio) was 0·51 per protocol (95% CI 0·20-0·70; log-rank p=0·0042) and 0·39 by mITT (0·04-0·62; p=0·033); vaccine efficacy (1-risk ratio) was 0·24 per-protocol (0·02-0·41; p=0·031) and 0·22 mITT (0·01-0·39; p=0·041).
Interpretation: A three-dose regimen of PfSPZ Vaccine was safe, well tolerated, and conferred 51% vaccine efficacy against intense natural P falciparum transmission, similar to 52% vaccine efficacy reported for a five-dose regimen in a previous trial.
Funding: US National Institute of Allergy and Infectious Diseases, National Institutes of Health, Sanaria.
Translation: For the French translation of the abstract see Supplementary Materials section.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests YA, ERJ, TM, NK, BKLS, PFB, TLR, and SLH are salaried, full-time employees of Sanaria, the developer and sponsor of Sanaria PfSPZ Vaccine. All other authors declare no competing interests.
Figures

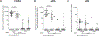

Comment in
-
Assessment of experimental malaria vaccine induced protection in pre-exposed populations.Lancet Infect Dis. 2022 Mar;22(3):305-307. doi: 10.1016/S1473-3099(21)00359-5. Epub 2021 Nov 18. Lancet Infect Dis. 2022. PMID: 34801111 No abstract available.
References
-
- WHO. World Malaria Report 2020. Geneva: World Health Organization, 2020.
-
- Dicko A, Sagara I, Diemert D, et al. Year-to-year variation in the age-specific incidence of clinical malaria in two potential vaccine testing sites in Mali with different levels of malaria transmission intensity. Am J Trop Med Hyg 2007; 77(6): 1028–33. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

