Rescuing activity of oxygen-damaged pyruvate formate-lyase by a spare part protein
- PMID: 34801558
- PMCID: PMC8683613
- DOI: 10.1016/j.jbc.2021.101423
Rescuing activity of oxygen-damaged pyruvate formate-lyase by a spare part protein
Abstract
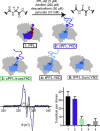
Pyruvate formate-lyase (PFL) is a glycyl radical enzyme (GRE) that converts pyruvate and coenzyme A into acetyl-CoA and formate in a reaction that is crucial to the primary metabolism of many anaerobic bacteria. The glycyl radical cofactor, which is posttranslationally installed by a radical S-adenosyl-L-methionine (SAM) activase, is a simple and effective catalyst, but is also susceptible to oxidative damage in microaerobic environments. Such damage occurs at the glycyl radical cofactor, resulting in cleaved PFL (cPFL). Bacteria have evolved a spare part protein termed YfiD that can be used to repair cPFL. Previously, we obtained a structure of YfiD by NMR spectroscopy and found that the N-terminus of YfiD was disordered and that the C-terminus of YfiD duplicates the structure of the C-terminus of PFL, including a β-strand that is not removed by the oxygen-induced cleavage. We also showed that cPFL is highly susceptible to proteolysis, suggesting that YfiD rescue of cPFL competes with protein degradation. Here, we probe the mechanism by which YfiD can bind and restore activity to cPFL through enzymatic and spectroscopic studies. Our data show that the disordered N-terminal region of YfiD is important for YfiD glycyl radical installation but not for catalysis, and that the duplicate β-strand does not need to be cleaved from cPFL for YfiD to bind. In fact, truncation of this PFL region prevents YfiD rescue. Collectively our data suggest the molecular mechanisms by which YfiD activation is precluded both when PFL is not damaged and when it is highly damaged.
Keywords: bacterial metabolism; cofactor repair; electron paramagnetic resonance (EPR) spectroscopy; enzyme inactivation; glycyl radical enzyme; isothermal titration calorimetry (ITC); oxygen-sensitive enzymes; protein complex; radical chemistry; spare part protein.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures






References
-
- Sun X., Ollagnier S., Schmidt P.P., Atta M., Mulliez E., Lepape L., Eliasson R., Graslund A., Fontecave M., Reichard P., Sjoberg B.M. The free radical of the anaerobic ribonucleotide reductase from Escherichia coli is at glycine 681. J. Biol. Chem. 1996;271:6827–6831. - PubMed
-
- Leuthner B., Leutwein C., Schulz H., Horth P., Haehnel W., Schiltz E., Schagger H., Heider J. Biochemical and genetic characterization of benzylsuccinate synthase from Thauera aromatica: A new glycyl radical enzyme catalysing the first step in anaerobic toluene metabolism. Mol. Microbiol. 1998;28:615–628. - PubMed
-
- Levin B.J., Balskus E.P. Discovering radical-dependent enzymes in the human gut microbiota. Curr. Opin. Chem. Biol. 2018;47:86–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

