Identification of Sputum Biomarkers Predictive of Pulmonary Exacerbations in COPD
- PMID: 34801592
- PMCID: PMC9131049
- DOI: 10.1016/j.chest.2021.10.049
Identification of Sputum Biomarkers Predictive of Pulmonary Exacerbations in COPD
Abstract
Background: Improved understanding of the pathways associated with airway pathophysiologic features in COPD will identify new predictive biomarkers and novel therapeutic targets.
Research question: Which physiologic pathways are altered in the airways of patients with COPD and will predict exacerbations?
Study design and methods: We applied a mass spectrometric panel of metabolomic biomarkers related to mucus hydration and inflammation to sputa from the multicenter Subpopulations and Intermediate Outcome Measures in COPD Study. Biomarkers elevated in sputa from patients with COPD were evaluated for relationships to measures of COPD disease severity and their ability to predict future exacerbations.
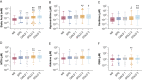
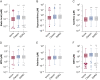
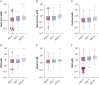
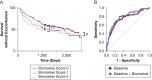
Results: Sputum supernatants from 980 patients were analyzed: 77 healthy nonsmokers, 341 smokers with preserved spirometry, and 562 patients with COPD (178 with Global Initiative on Chronic Obstructive Lung Disease [GOLD] stage 1 disease, 303 with GOLD stage 2 disease, and 81 with GOLD stage 3 disease) were analyzed. Biomarkers from multiple pathways were elevated in COPD and correlated with sputum neutrophil counts. Among the most significant analytes (false discovery rate, 0.1) were sialic acid, hypoxanthine, xanthine, methylthioadenosine, adenine, and glutathione. Sialic acid and hypoxanthine were associated strongly with measures of disease severity, and elevation of these biomarkers was associated with shorter time to exacerbation and improved prediction models of future exacerbations.
Interpretation: Biomarker evaluation implicated pathways involved in mucus hydration, adenosine metabolism, methionine salvage, and oxidative stress in COPD airway pathophysiologic characteristics. Therapies that target these pathways may be of benefit in COPD, and a simple model adding sputum-soluble phase biomarkers improves prediction of pulmonary exacerbations.
Trial registry: ClinicalTrials.gov; No.: NCT01969344; URL: www.
Clinicaltrials: gov.
Keywords: adenosine; glutathione; inflammation; metabolomics; methionine salvage; mucus.
Copyright © 2021 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Paone G., Leone V., Conti V., et al. Blood and sputum biomarkers in COPD and asthma: a review. Eur Rev Med Pharmacol Sci. 2016;20(4):698–708. - PubMed
-
- Beeh K.M., Beier J., Koppenhoefer N., Buhl R. Increased glutathione disulfide and nitrosothiols in sputum supernatant of patients with stable COPD. Chest. 2004;126(4):1116–1122. - PubMed
-
- Kinnula V.L., Ilumets H., Myllarniemi M., Sovijarvi A., Rytila P. 8-Isoprostane as a marker of oxidative stress in nonsymptomatic cigarette smokers and COPD. Eur Respir J. 2007;29(1):51–55. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HHSN268200900019C/HL/NHLBI NIH HHS/United States
- P01 HL110873/HL/NHLBI NIH HHS/United States
- P50 HL107168/HL/NHLBI NIH HHS/United States
- HHSN268200900015C/HL/NHLBI NIH HHS/United States
- HHSN268200900016C/HL/NHLBI NIH HHS/United States
- R01 HL148215/HL/NHLBI NIH HHS/United States
- U01 HL137880/HL/NHLBI NIH HHS/United States
- UH3 HL123645/HL/NHLBI NIH HHS/United States
- HHSN268200900018C/HL/NHLBI NIH HHS/United States
- HHSN268200900013C/HL/NHLBI NIH HHS/United States
- R01 AI129958/AI/NIAID NIH HHS/United States
- HHSN268200900014C/HL/NHLBI NIH HHS/United States
- P01 HL108808/HL/NHLBI NIH HHS/United States
- P30 DK065988/DK/NIDDK NIH HHS/United States
- U24 HL141762/HL/NHLBI NIH HHS/United States
- P30 ES010126/ES/NIEHS NIH HHS/United States
- R01 HL136961/HL/NHLBI NIH HHS/United States
- HHSN268200900017C/HL/NHLBI NIH HHS/United States
- HHSN268200900020C/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

