Crystal structure of semisynthetic obelin-v
- PMID: 34802167
- PMCID: PMC8819848
- DOI: 10.1002/pro.4244
Crystal structure of semisynthetic obelin-v
Abstract
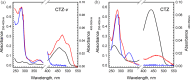
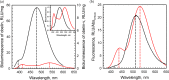
Coelenterazine-v (CTZ-v), a synthetic derivative with an additional benzyl ring, yields a bright bioluminescence of Renilla luciferase and its "yellow" mutant with a significant shift in the emission spectrum toward longer wavelengths, which makes it the substrate of choice for deep tissue imaging. Although Ca2+ -regulated photoproteins activated with CTZ-v also display red-shifted light emission, in contrast to Renilla luciferase their bioluminescence activities are very low, which makes photoproteins activated by CTZ-v unusable for calcium imaging. Here, we report the crystal structure of Ca2+ -regulated photoprotein obelin with 2-hydroperoxycoelenterazine-v (obelin-v) at 1.80 Å resolution. The structures of obelin-v and obelin bound with native CTZ revealed almost no difference; only the minor rearrangement in hydrogen-bond pattern and slightly increased distances between key active site residues and some atoms of 2-hydroperoxycoelenterazine-v were found. The fluorescence quantum yield (ΦFL ) of obelin bound with coelenteramide-v (0.24) turned out to be even higher than that of obelin with native coelenteramide (0.19). Since both obelins are in effect the enzyme-substrate complexes containing the 2-hydroperoxy adduct of CTZ-v or CTZ, we reasonably assume the chemical reaction mechanisms and the yields of the reaction products (ΦR ) to be similar for both obelins. Based on these findings we suggest that low bioluminescence activity of obelin-v is caused by the low efficiency of generating an electronic excited state (ΦS ). In turn, the low ΦS value as compared to that of native CTZ might be the result of small changes in the substrate microenvironment in the obelin-v active site.
Keywords: analog; bioluminescence; coelenterazine; coelenterazine-v; obelin; photoprotein; protein structure.
© 2021 The Protein Society.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



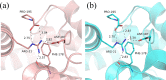

Similar articles
-
Crystal structure of semi-synthetic obelin-v after calcium induced bioluminescence implies coelenteramine as the main reaction product.Sci Rep. 2022 Nov 15;12(1):19613. doi: 10.1038/s41598-022-24117-5. Sci Rep. 2022. PMID: 36379962 Free PMC article.
-
Violet bioluminescence and fast kinetics from W92F obelin: structure-based proposals for the bioluminescence triggering and the identification of the emitting species.Biochemistry. 2003 May 27;42(20):6013-24. doi: 10.1021/bi027258h. Biochemistry. 2003. PMID: 12755603
-
Bioluminescent Properties of Semi-Synthetic Obelin and Aequorin Activated by Coelenterazine Analogues with Modifications of C-2, C-6, and C-8 Substituents.Int J Mol Sci. 2020 Jul 30;21(15):5446. doi: 10.3390/ijms21155446. Int J Mol Sci. 2020. PMID: 32751691 Free PMC article.
-
Exploring Bioluminescence Function of the Ca2+ -regulated Photoproteins with Site-directed Mutagenesis.Photochem Photobiol. 2019 Jan;95(1):8-23. doi: 10.1111/php.12945. Epub 2018 Jun 22. Photochem Photobiol. 2019. PMID: 29855041 Review.
-
Mutants of Ca2+ -regulated Photoprotein Obelin for Site-specific Conjugation.Photochem Photobiol. 2017 Mar;93(2):553-557. doi: 10.1111/php.12712. Epub 2017 Mar 8. Photochem Photobiol. 2017. PMID: 28063150 Review.
Cited by
-
Crystal structure of semi-synthetic obelin-v after calcium induced bioluminescence implies coelenteramine as the main reaction product.Sci Rep. 2022 Nov 15;12(1):19613. doi: 10.1038/s41598-022-24117-5. Sci Rep. 2022. PMID: 36379962 Free PMC article.
-
Fast kinetics of photoprotein emitting species.Sci Rep. 2025 Aug 27;15(1):31577. doi: 10.1038/s41598-025-17152-5. Sci Rep. 2025. PMID: 40866465 Free PMC article.
References
-
- Shimomura O. Bioluminescence: Chemical principles and methods. Singapore: World Scientific Publishing Co Pte Ltd, 2006.
-
- Oba Y, Kato S, Ojika M, Inouye S. Biosynthesis of coelenterazine in the deep‐sea copepod, Metridia pacifica . Biochem Biophys Res Commun. 2009;390:684–688. - PubMed
-
- Matthews JC, Hori K, Cormier MJ. Purification and properties of Renilla reniformis luciferase. Biochemistry. 1977;16:85–91. - PubMed
-
- Verhaegent M, Christopoulos TK. Recombinant Gaussia luciferase. Overexpression, purification, and analytical application of a bioluminescent reporter for DNA hybridization. Anal Chem. 2002;74:4378–4385. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

