Production of Long-Acting CNGRC-CPG2 Fusion Proteins: New Derivatives to Overcome Drug Immunogenicity of Ligand-Directed Enzyme Prodrug Therapy for Targeted Cancer Treatment
- PMID: 34802309
- PMCID: PMC8606725
- DOI: 10.1177/15330338211057371
Production of Long-Acting CNGRC-CPG2 Fusion Proteins: New Derivatives to Overcome Drug Immunogenicity of Ligand-Directed Enzyme Prodrug Therapy for Targeted Cancer Treatment
Erratum in
-
Corrigendum.Technol Cancer Res Treat. 2022 Jan-Dec;21:15330338221106823. doi: 10.1177/15330338221106823. Technol Cancer Res Treat. 2022. PMID: 35765742 Free PMC article. No abstract available.
Abstract
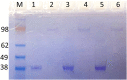
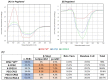
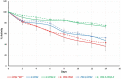
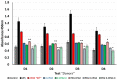
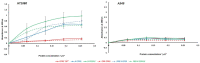
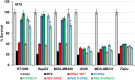
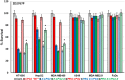
Objectives: Aminopeptidase N (APN) is an enzyme highly expressed in metastatic cancers and could be used in targeted cancer therapy. Our previous work showed the successful construction of CNGRC-carboxypeptidase G2 (CPG2) and CNGRC-CPG2-CNGRC fusion proteins. Our conjugates and prodrugs were effective in targeting high APN-expressing cancer cells. In the present study, we aim to produce long-acting fusion proteins to overcome 2 of the main drawbacks of antibody-directed enzyme prodrug therapy. Methods: N-terminal and N-, C-terminal fusion CPG2, CNGRC-CPG2, and CNGRC-CPG2-CNGRC, respectively, were PEGylated using polyethylene glycol (PEG) maleimide (40K). We examined the effect of PEGylation on the therapeutic efficacy of the new products. The resulting PEGylated fusion proteins were tested for their stability, ex vivo immunotoxicity, binding capacity to their target on high HT1080, and low A549 APN-expressing cells. The catalytic activity of the resulting PEGylated fusion CPG2 proteins was investigated. Pro-drug "ZD2767P" cytotoxic effect in association with PEG CPG2-CNGRC fusion proteins on cancer cells was studied. Results: Our work demonstrated that the properties of the PEGylated single-fused proteins were significantly improved over that of un-PEGylated fused CPG2, and its kinetic activity and APN-binding affinity were not negatively affected by the PEGylation. Significantly, The PEGylated single-fused CPG2 had lower immunogenicity than the un-PEGylated CPG2. Our results, however, were different in the case of the PEGylated double-fused CPG2. Although its stability in human serum under physiological conditions was not significantly affected, the kinetic activity and its binding affinity to their cellular marker (APN) were substantially reduced. When the study was performed with high and low APN-expressing cancer cell lines, using the prodrug ZD2767p, the PEGylated fusion CPG2 demonstrated cancer cell killing effects. Conclusion: We have successfully produced PEGylated-CNGRC-CPG2, which is bioactive and with lower immunogenicity in ligand-directed enzyme prodrug therapy for cancer treatment.
Keywords: PEGylated CPG2 conjugates; aminopeptidase N; immunogenicity; ligand-directed enzyme prodrug therapy; long-acting drugs; targeted cancer therapy.
Figures

Similar articles
-
In vitro studies on CNGRC-CPG2 fusion proteins for ligand-directed enzyme prodrug therapy for targeted cancer therapy.Oncotarget. 2020 Feb 11;11(6):619-633. doi: 10.18632/oncotarget.27478. eCollection 2020 Feb 11. Oncotarget. 2020. PMID: 32110281 Free PMC article.
-
Targeted antitumor prodrug therapy using CNGRC-yCD fusion protein in combination with 5-fluorocytosine.J Biomed Sci. 2016 Jan 22;23:15. doi: 10.1186/s12929-016-0227-6. J Biomed Sci. 2016. PMID: 26801910 Free PMC article.
-
Production of "biobetter" glucarpidase variants to improve drug detoxification and antibody directed enzyme prodrug therapy for cancer treatment.Eur J Pharm Sci. 2019 Jan 15;127:79-91. doi: 10.1016/j.ejps.2018.10.014. Epub 2018 Oct 19. Eur J Pharm Sci. 2019. PMID: 30343151
-
Glucarpidase (carboxypeptidase G2): Biotechnological production, clinical application as a methotrexate antidote, and placement in targeted cancer therapy.Biomed Pharmacother. 2023 Oct;166:115292. doi: 10.1016/j.biopha.2023.115292. Epub 2023 Aug 12. Biomed Pharmacother. 2023. PMID: 37579696 Review.
-
Antibody-directed enzyme prodrug therapy (ADEPT) with mustard prodrugs.Anticancer Drug Des. 1995 Jul;10(5):361-72. Anticancer Drug Des. 1995. PMID: 7639927 Review.
Cited by
-
Prodrug-carboxypeptidase G2 therapy: certain concerns on carboxypeptidase G2.Front Pharmacol. 2025 Jun 12;16:1560834. doi: 10.3389/fphar.2025.1560834. eCollection 2025. Front Pharmacol. 2025. PMID: 40575779 Free PMC article. No abstract available.
References
-
- Carter PJ. Introduction to current and future protein therapeutics: a protein engineering perspective. Exp Cell Res. 2011;317(9):1261-1269. - PubMed
-
- Turecek PL, Bossard MJ, Schoetens F, Ivens IA. PEGylation of biopharmaceuticals: a review of chemistry and nonclinical safety information of approved drugs. J Pharm Sci. 2016;105(2):460-475. - PubMed
-
- Mishra P, Nayak B, Dey RK. PEGylation in anti-cancer therapy: an overview. Asian J Pharm Sci. 2016;11(3):337-348.
-
- Reichert C, Borchard G. Noncovalent PEGylation, An innovative subchapter in the field of protein modification. J Pharm Sci. 2016;105(2):386-390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

