Descriptive evaluation of antibody responses to severe acute respiratory coronavirus virus 2 (SARS-CoV-2) infection in plasma and gingival crevicular fluid in a nursing home cohort-Arkansas, June-August 2020
- PMID: 34802478
- PMCID: PMC9379264
- DOI: 10.1017/ice.2021.484
Descriptive evaluation of antibody responses to severe acute respiratory coronavirus virus 2 (SARS-CoV-2) infection in plasma and gingival crevicular fluid in a nursing home cohort-Arkansas, June-August 2020
Abstract
Objective: To characterize and compare severe acute respiratory coronavirus virus 2 (SARS-CoV-2)-specific immune responses in plasma and gingival crevicular fluid (GCF) from nursing home residents during and after natural infection.
Design: Prospective cohort.
Setting: Nursing home.
Participants: SARS-CoV-2-infected nursing home residents.
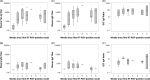
Methods: A convenience sample of 14 SARS-CoV-2-infected nursing home residents, enrolled 4-13 days after real-time reverse transcription polymerase chain reaction diagnosis, were followed for 42 days. After diagnosis, plasma SARS-CoV-2-specific pan-Immunoglobulin (Ig), IgG, IgA, IgM, and neutralizing antibodies were measured at 5 time points, and GCF SARS-CoV-2-specific IgG and IgA were measured at 4 time points.
Results: All participants demonstrated immune responses to SARS-CoV-2 infection. Among 12 phlebotomized participants, plasma was positive for pan-Ig and IgG in all 12 participants. Neutralizing antibodies were positive in 11 participants; IgM was positive in 10 participants, and IgA was positive in 9 participants. Among 14 participants with GCF specimens, GCF was positive for IgG in 13 participants and for IgA in 12 participants. Immunoglobulin responses in plasma and GCF had similar kinetics; median times to peak antibody response were similar across specimen types (4 weeks for IgG; 3 weeks for IgA). Participants with pan-Ig, IgG, and IgA detected in plasma and GCF IgG remained positive throughout this evaluation, 46-55 days after diagnosis. All participants were viral-culture negative by the first detection of antibodies.
Conclusions: Nursing home residents had detectable SARS-CoV-2 antibodies in plasma and GCF after infection. Kinetics of antibodies detected in GCF mirrored those from plasma. Noninvasive GCF may be useful for detecting and monitoring immunologic responses in populations unable or unwilling to be phlebotomized.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

