Immunogenicity of full-length P. vivax rPvs48/45 protein formulations in BALB/c mice
- PMID: 34802791
- PMCID: PMC9109962
- DOI: 10.1016/j.vaccine.2021.11.036
Immunogenicity of full-length P. vivax rPvs48/45 protein formulations in BALB/c mice
Abstract
Background: Pvs48/45 is a Plasmodium vivax gametocyte surface protein involved in the parasite fertilization process. Previous studies showed that Pvs48/45 proteins expressed in Escherichia coli (E. coli) and Chinese hamster ovary (CHO) cells were highly immunoreactive with sera from malaria-endemic areas and highly immunogenic in animal models. Here the immunogenicity in mice of three different vaccine formulations was compared.
Methods: Recombinant (r) Pvs48/45 proteins were expressed in E. coli and CHO, purified, formulated in Alhydrogel, GLA-SE and Montanide ISA-51 adjuvants and used to immunize BALB/c mice. Animals were immunized on days 0, 20 and 40, and serum samples were collected for serological analyses of specific antibody responses using ELISA and immunofluorescence (IFAT). Additionally, ex-vivo transmission-reducing activity (TRA) of sera on P. vivax gametocyte-infected human blood fed to Anopheles albimanus in direct membrane feeding assays (DMFA) was evaluated.
Results: Most immunized animals seroconverted after the first immunization, and some developed antibody peaks of 106 with all adjuvants. However, the three adjuvant formulations induced different antibody responses and TRA efficacy. While GLA-SE formulations of both proteins induced similar antibody profiles, Montanide ISA-51 formulations resulted in higher and longer-lasting antibody titers with CHO-rPvs48/45 than with the E. coli formulation. Although the CHO protein formulated in Alhydrogel generated a high initial antibody peak, antibody responses to both proteins rapidly waned. Likewise, anti-Pvs48/45 antibodies displayed differential recognition of the parasite proteins in IFAT and ex vivo blockade of parasite transmission to mosquitoes. The CHO-rPvs48/45 formulated in Montanide ISA-51 induced the most effective ex vivo parasite blockage.
Conclusions: Three out of six vaccine formulations elicited antibodies with ex vivo TRA. The CHO-rPvs48/45 Montanide ISA-51 formulation induced the most stable antibody response, recognizing the native protein and the most robust ex vivo TRA. These results encourage further testing of the vaccine potential of this protein.
Keywords: Gametocytes; Immunogenicity; Malaria; Plasmodium vivax; Pvs48/45; Transmission Reducing Activity; Transmission blocking vaccine; Vaccines.
Copyright © 2021. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper: [Myriam Arevalo-Herrera reports financial support was provided by NIH, NIAID.].
Figures

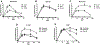


References
-
- Feachem RGA, Chen I, Akbari O, Bertozzi-Villa A, Bhatt S, Binka F, et al. Malaria eradication within a generation: ambitious, achievable, and necessary. Lancet. 2019;394:1056–112. - PubMed
-
- WHO. Malaria progress report. Geneva: WHO; 2020.
-
- WHO. Tackling antimalarial drug resistance In: www.who.int/docs/default-source/malaria/drug-resistance/who-ucn-gmp-2020-07... editor.2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

