BNT162b2 mRNA COVID-19 vaccine Reactogenicity: The key role of immunity
- PMID: 34802792
- PMCID: PMC8580836
- DOI: 10.1016/j.vaccine.2021.10.074
BNT162b2 mRNA COVID-19 vaccine Reactogenicity: The key role of immunity
Abstract
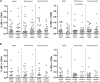
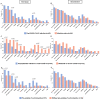
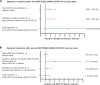
We examined the impact of pre-existing SARS-CoV-2-specific cellular immunity on BNT162b2 mRNA COVID-19 vaccine reactogenicity. Of 96 healthcare workers (HCWs), 76% reported any vaccine reaction (first dose: 70%, second dose: 67%), none of which was severe. Following first dose, systemic reactions were significantly more frequent among HCWs with past infection than in infection-naïve individuals, and among HCWs with pre-existing cellular immunity than in those without it. The rate of systemic reactions after second dose was 1.7 and 2.0-times higher than after first dose among infection-naïve HCWs and those without pre-existing cellular immunity, respectively. Levels of SARS-CoV-2-specific T-cells before vaccination were higher in HCWs with systemic reactions after the first dose than in those without them. BNT162b2 vaccine reactogenicity after first dose is attributable to pre-existing cellular immunity elicited by prior COVID-19 or cross-reactivity. Reactogenicity following second dose suggests an immunity-boosting effect. Overall, these data may reduce negative attitudes towards COVID-19 vaccines. Study Registration. The study was registered on clinicaltrials.gov, NCT04402827.
Keywords: BNT162b2 mRNA vaccine; Booster effect; COVID-19; Reactogenicity; SARS-CoV-2; T-cell responses.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939–949. doi: 10.1016/S1473-3099(21)00224-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

