A Telemedicine-Guided Self-Collection Approach for PCR-Based SARS-CoV-2 Testing: Comparative Study
- PMID: 34803022
- PMCID: PMC8729873
- DOI: 10.2196/32564
A Telemedicine-Guided Self-Collection Approach for PCR-Based SARS-CoV-2 Testing: Comparative Study
Abstract
Background: Large-scale, polymerase chain reaction (PCR)-based SARS-CoV-2 testing is expensive, resource intensive, and time consuming. A self-collection approach is a probable alternative; however, its feasibility, cost, and ability to prevent infections need to be evaluated.
Objective: This study aims to compare an innovative self-collection approach with a regular SARS-CoV-2 testing strategy in a large European industrial manufacturing site.
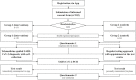
Methods: The feasibility of a telemedicine-guided PCR-based self-collection approach was assessed for 150 employees (intervention group) and compared with a regular SARS-CoV-2 testing approach used for 143 employees (control group). Acceptance, ergonomics, and efficacy were evaluated using a software application. A simulation model was implemented to evaluate the effectiveness. An interactive R shiny app was created to enable customized simulations.
Results: The test results were successfully communicated to and interpreted without uncertainty by 76% (114/150) and 76.9% (110/143) of the participants in the intervention and control groups, respectively (P=.96). The ratings for acceptability, ergonomics, and efficacy among intervention group participants were noninferior when compared to those among control group participants (acceptability: 71.6% vs 37.6%; ergonomics: 88.1% vs 74.5%; efficacy: 86.4% vs 77.5%). The self-collection approach was found to be less time consuming (23 min vs 38 min; P<.001). The simulation model indicated that both testing approaches reduce the risk of infection, and the self-collection approach tends to be slightly less effective owing to its lower sensitivity.
Conclusions: The self-collection approach for SARS-CoV-2 diagnosis was found to be technically feasible and well rated in terms of acceptance, ergonomics, and efficacy. The simulation model facilitates the evaluation of test effectiveness; nonetheless, considering context specificity, appropriate adaptation by companies is required.
Keywords: COVID-19; SARS-CoV-2; self-sampling; simulation model; telemedicine; test strategy effectiveness.
©Silvia Würstle, Johanna Erber, Michael Hanselmann, Dieter Hoffmann, Stanislas Werfel, Svenja Hering, Simon Weidlich, Jochen Schneider, Ralf Franke, Michael Maier, Andreas G Henkel, Roland M Schmid, Ulrike Protzer, Michael Laxy, Christoph D Spinner. Originally published in JMIR Formative Research (https://formative.jmir.org), 04.01.2022.
Conflict of interest statement
Conflicts of Interest: CDS reports grants, personal fees, and nonfinancial support from AbbVie, Apeiron, Gilead Sciences, Janssen-Cilag, GSK/ViiV Healthcare, and MSD; grants and personal fees from BBraun and Eli Lilly; grants from Cepheid; and personal fees from Formycon—outside the submitted work. S Weidlich reports personal fees and nonfinancial support from Gilead Sciences and Janssen-Cilag outside the submitted work. All other authors declare that they have no conflicts of interest.
Figures
References
-
- Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance, 2 March 2020. World Health Organization. 2020. [2021-12-15]. https://apps.who.int/iris/handle/10665/331329 .
-
- Fitzpatrick MC, Pandey A, Wells CR, Sah P, Galvani AP. Buyer beware: inflated claims of sensitivity for rapid COVID-19 tests. Lancet. 2021 Jan 02;397(10268):24–25. doi: 10.1016/S0140-6736(20)32635-0. http://europepmc.org/abstract/MED/33333002 S0140-6736(20)32635-0 - DOI - PMC - PubMed
-
- Würstle S, Spinner CD, Voit F, Hoffmann D, Hering S, Weidlich S, Schneider J, Zink A, Treiber M, Iakoubov R, Schmid RM, Protzer U, Erber J. Self-sampling versus health care professional-guided swab collection for SARS-CoV-2 testing. Infection. 2021 Oct;49(5):927–934. doi: 10.1007/s15010-021-01614-9. http://europepmc.org/abstract/MED/33970430 10.1007/s15010-021-01614-9 - DOI - PMC - PubMed
-
- Wehrhahn MC, Robson J, Brown S, Bursle E, Byrne S, New D, Chong S, Newcombe JP, Siversten T, Hadlow N. Self-collection: An appropriate alternative during the SARS-CoV-2 pandemic. J Clin Virol. 2020 Jul;128:104417. doi: 10.1016/j.jcv.2020.104417. http://europepmc.org/abstract/MED/32403007 S1386-6532(20)30159-1 - DOI - PMC - PubMed
-
- Tu Y, Jennings R, Hart B, Cangelosi GA, Wood RC, Wehber K, Verma P, Vojta D, Berke EM. Swabs collected by patients or health care workers for SARS-CoV-2 testing. N Engl J Med. 2020 Jul 30;383(5):494–496. doi: 10.1056/NEJMc2016321. http://europepmc.org/abstract/MED/32492294 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

