Influence of timing of maternal antibiotic administration during caesarean section on infant microbial colonisation: a randomised controlled trial
- PMID: 34803023
- PMCID: PMC9380480
- DOI: 10.1136/gutjnl-2021-324767
Influence of timing of maternal antibiotic administration during caesarean section on infant microbial colonisation: a randomised controlled trial
Abstract
Objective: Revised guidelines for caesarean section (CS) advise maternal antibiotic administration prior to skin incision instead of after umbilical cord clamping, unintentionally exposing the infant to antibiotics antenatally. We aimed to investigate if timing of intrapartum antibiotics contributes to the impairment of microbiota colonisation in CS born infants.
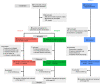
Design: In this randomised controlled trial, women delivering via CS received antibiotics prior to skin incision (n=20) or after umbilical cord clamping (n=20). A third control group of vaginally delivering women (n=23) was included. Faecal microbiota was determined from all infants at 1, 7 and 28 days after birth and at 3 years by 16S rRNA gene sequencing and whole-metagenome shotgun sequencing.
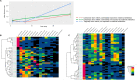
Results: Compared with vaginally born infants, profound differences were found in microbial diversity and composition in both CS groups in the first month of life. A decreased abundance in species belonging to the genera Bacteroides and Bifidobacterium was found with a concurrent increase in members belonging to the phylum Proteobacteria. These differences could not be observed at 3 years of age. No statistically significant differences were observed in taxonomic and functional composition of the microbiome between both CS groups at any of the time points.
Conclusion: We confirmed that microbiome colonisation is strongly affected by CS delivery. Our findings suggest that maternal antibiotic administration prior to CS does not result in a second hit on the compromised microbiome. Future, larger studies should confirm that antenatal antibiotic exposure in CS born infants does not aggravate colonisation impairment and impact long-term health.
Keywords: antibiotics; infant gut; intestinal microbiology; paediatric gastroenterology.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the International Committee of Medical Journal Editors (ICMJE) uniform disclosure form at http://www.icmje.org/conflicts-of-interest/. Authors AE, ST, JK and GR are employees of Danone Nutricia Research which partially funded the Clinical Trial. TdM has served as a speaker for Danone Nutricia Research and Mead Johnson. NdB has served as a speaker for AbbVie and MSD. He has served as consultant and/or principal investigator for TEVA Pharma BV and Takeda. He has received a (unrestricted) research grant from Dr. Falk, TEVA Pharma BV and Takeda. The other authors have no financial disclosures that would be a potential conflict of interest.
Figures
Comment in
-
Maternal antibiotic exposure during pregnancy and risk of IBD in offspring: a population-based cohort study.Gut. 2023 Apr;72(4):804-805. doi: 10.1136/gutjnl-2022-327724. Epub 2022 Jun 8. Gut. 2023. PMID: 35676084 Free PMC article. No abstract available.
References
-
- Bartlett JG, Chang TW, Gurwith M. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med 1978;298:531–4. - PubMed
-
- Esaiassen E, Fjalstad JW, Juvet LK. Antibiotic exposure in neonates and early adverse outcomes: a systematic review and meta-analysis. J Antimicrob Chemother 2017;72:1858–70. - PubMed
-
- Galazzo G, van Best N, Bervoets L. Development of the microbiota and associations with birth mode, diet, and atopic disorders in a longitudinal analysis of stool samples, collected from infancy through early childhood. Gastroenterology 2020;158:1584–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
