Establishing a technique for isolation and characterization of human periodontal ligament derived mesenchymal stem cells
- PMID: 34803321
- PMCID: PMC8589598
- DOI: 10.1016/j.sdentj.2020.04.007
Establishing a technique for isolation and characterization of human periodontal ligament derived mesenchymal stem cells
Abstract
Mesenchymal stem cells (MSCs) are extensively used in tissue regenerative procedures. One source of MSCs is the periodontal ligament (PDL) of teeth. Isolation of MSCs from extracted teeth is reasonably simple, being less invasive and presenting fewer ethical concerns than does the harvesting of MSC's from other sites. The objectives of this study were to isolate and characterize the PDL stem cells (PDLSC) from healthy adults' extracted teeth and then to characterize them by comparing them with bone-marrow derived MSCs (BMMSC).
Methods: The PDL tissue was scraped from the roots of freshly extracted teeth to enzymatically digest using collagenase. The cells were sub-cultured. Flow-cytometric analysis for the MSC surface-markers CD105, CD73, CD166, CD90, CD34, CD45 and HLA-DR was performed. To confirm the phenotype, total RNA was extracted to synthesize cDNA and which was then subjected to RT-PCR. The gene-expression for Oct4A, Sox2, NANOG and GAPDH was determined by gel-electrophoresis. To assess their multilineage potential, cells were cultured with osteogenic, chondrogenic and adipogenic medium and then stained by Alizarin-red, Alcian-blue and Oil-Red-O respectively. MSCs from the bone-marrow were processed similarly to serve as controls.
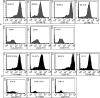
Results: The cells isolated from extracted teeth expanded successfully. On flow-cytometric analysis, the cells were positive for CD73, CD90, CD105, CD166 and negative for CD34, CD45 and HLA-DR. The PDLSCs expressed Oct4A, Sox2, and NANOG mRNA with GAPDH expression. Cells cultured in the osteogenic, chondrogenic and adipogenic media stained positive for Alizarin-red, Alcian-blue and Oil- Red-O respectively. The surface marker expression and the trilineage differentiation characteristics were comparable to those of the BMMSCs.
Conclusions: The periodontal ligament tissue of extracted teeth is a potential source of therapeutically useful MSCs. Harvesting them is not invasive and are a promising source of MSC as the PDLSCs showed characteristics similar to those of the highly regarded MSC's derived from bone-marrow.
Keywords: Adult stem cells; Cell differentiation; Mesenchymal stem cells; Periodontal ligament; Periodontal ligament stem cells; Pluripotency; Progenitor cells; Regeneration; Stem cells; Tissue engineering.
© 2020 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Comparison of Cellular and Differentiation Characteristics of Mesenchymal Stem Cells Derived from Human Gingiva and Periodontal Ligament.J Int Soc Prev Community Dent. 2022 Apr 8;12(2):235-244. doi: 10.4103/jispcd.JISPCD_259_21. eCollection 2022 Mar-Apr. J Int Soc Prev Community Dent. 2022. PMID: 35462740 Free PMC article.
-
Assessment of Surface Markers Derived from Human Periodontal Ligament Stem Cells: An In Vitro Study.J Dent (Tehran). 2016 Sep;13(5):325-332. J Dent (Tehran). 2016. PMID: 28127326 Free PMC article.
-
A comparative study on immunophenotypic characterization and osteogenic differentiation of human mesenchymal stromal cells derived from periodontal ligament and gingiva.J Periodontol. 2020 Sep;91(9):1194-1202. doi: 10.1002/JPER.19-0535. Epub 2020 Jul 17. J Periodontol. 2020. PMID: 31960428
-
Periodontal Ligament Stem Cells: Current Knowledge and Future Perspectives.Stem Cells Dev. 2019 Aug 1;28(15):995-1003. doi: 10.1089/scd.2019.0025. Epub 2019 May 20. Stem Cells Dev. 2019. PMID: 31017047 Review.
-
Oral Mesenchymal Stem/Progenitor Cells: The Immunomodulatory Masters.Stem Cells Int. 2020 Feb 25;2020:1327405. doi: 10.1155/2020/1327405. eCollection 2020. Stem Cells Int. 2020. PMID: 32184830 Free PMC article. Review.
Cited by
-
Mesenchymal Stromal Cells Derived from Dental Tissues: Immunomodulatory Properties and Clinical Potential.Int J Mol Sci. 2024 Feb 6;25(4):1986. doi: 10.3390/ijms25041986. Int J Mol Sci. 2024. PMID: 38396665 Free PMC article. Review.
-
Mesenchymal Stem Cell-Derived Apoptotic Bodies: Biological Functions and Therapeutic Potential.Cells. 2022 Dec 1;11(23):3879. doi: 10.3390/cells11233879. Cells. 2022. PMID: 36497136 Free PMC article. Review.
-
Periodontal ligament stem cells as a promising therapeutic target for neural damage.Stem Cell Res Ther. 2022 Jun 21;13(1):273. doi: 10.1186/s13287-022-02942-9. Stem Cell Res Ther. 2022. PMID: 35729595 Free PMC article. Review.
-
The optimum oxygen level in hypoxic culture conditions of ligament derived stem cells: experimental research.Ann Med Surg (Lond). 2023 May 23;85(6):2689-2694. doi: 10.1097/MS9.0000000000000900. eCollection 2023 Jun. Ann Med Surg (Lond). 2023. PMID: 37363459 Free PMC article.
-
Enhanced Proliferative and Osteogenic Potential of Periodontal Ligament Stromal Cells.Biomedicines. 2023 May 3;11(5):1352. doi: 10.3390/biomedicines11051352. Biomedicines. 2023. PMID: 37239023 Free PMC article.
References
-
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D.S., Deans R.J., Keating A., Prockop D.J., Horwitz E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

