Melatonin Ameliorates Axonal Hypomyelination of Periventricular White Matter by Transforming A1 to A2 Astrocyte via JAK2/STAT3 Pathway in Septic Neonatal Rats
- PMID: 34803390
- PMCID: PMC8595063
- DOI: 10.2147/JIR.S337499
Melatonin Ameliorates Axonal Hypomyelination of Periventricular White Matter by Transforming A1 to A2 Astrocyte via JAK2/STAT3 Pathway in Septic Neonatal Rats
Abstract
Background: Astrocyte A1/A2 phenotypes may play differential role in the pathogenesis of periventricular white matter (PWM) damage in septic postnatal rats. This study aimed to determine whether melatonin (MEL) would improve the axonal hypomyelination through shifting A1 astrocytes towards A2.
Methods: One-day-old Sprague-Dawley rats were divided into control, LPS, and LPS+MEL groups. Immunofluorescence was performed to detect C1q, IL-1α, TNF-α, IBA1, GFAP, MAG, C3 and S100A10 immunoreactivity in the PWM of neonatal rats. Electron microscopy was conducted to observe alterations of axonal myelin sheath in the PWM; moreover, myelin protein expression was assessed using in situ hybridization. The effects of MEL on neurological function were evaluated by behavioral tests. In vitro, A1 astrocytes were induced by IL-1α, C1q and TNF-α, and following which the effect of MEL on C3 and S100A10 expression was determined by Western blot and immunofluorescence.
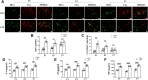
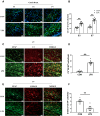
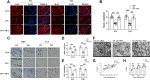
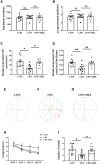
Results: At 1 and 3 days after LPS injection, IBA1+ microglia in the PWM were significantly increased in cell numbers which generated excess amounts of IL-1α, TNF-α, and C1q. The number of A1 astrocytes was significantly increased at 7-28d after LPS injection. In rats given MEL treatment, the number of A1 astrocytes was significantly decreased, but that of A2 astrocytes, PLP+, MBP+ and MAG+ cells was increased. By electron microscopy, ultrastructural features of axonal hypomyelination were attenuated by MEL. Furthermore, MEL improved neurological dysfunction as evaluated by different neurological tests. In vitro, MEL decreased the C3 significantly, and upregulated expression of S100A10 in primary astrocytes subjected to IL-1α, TNF-α and C1q treatment. Importantly, JAK2/STAT3 signaling pathway was found to be involved in modulation of A1/A2 phenotype transformation.
Conclusion: MEL effectively alleviates PWMD of septic neonatal rats, which is most likely through modulating astrocyte phenotypic transformation from A1 to A2 via the MT1/JAK2/STAT3 pathway.
Keywords: astrocyte; hypomyelination; melatonin; neuroprotection; sepsis.
© 2021 Jiang et al.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Complement C3a induces axonal hypomyelination in the periventricular white matter through activation of WNT/β-catenin signal pathway in septic neonatal rats experimentally induced by lipopolysaccharide.Brain Pathol. 2020 May;30(3):495-514. doi: 10.1111/bpa.12798. Epub 2019 Nov 6. Brain Pathol. 2020. PMID: 31622511 Free PMC article.
-
Melatonin Reduces Neuroinflammation and Improves Axonal Hypomyelination by Modulating M1/M2 Microglia Polarization via JAK2-STAT3-Telomerase Pathway in Postnatal Rats Exposed to Lipopolysaccharide.Mol Neurobiol. 2021 Dec;58(12):6552-6576. doi: 10.1007/s12035-021-02568-7. Epub 2021 Sep 28. Mol Neurobiol. 2021. PMID: 34585328 Free PMC article.
-
IL-1β induces hypomyelination in the periventricular white matter through inhibition of oligodendrocyte progenitor cell maturation via FYN/MEK/ERK signaling pathway in septic neonatal rats.Glia. 2016 Apr;64(4):583-602. doi: 10.1002/glia.22950. Epub 2015 Dec 17. Glia. 2016. PMID: 26678483
-
Bu Shen Yi Sui Capsule Promotes Myelin Repair by Modulating the Transformation of A1/A2 Reactive Astrocytes In Vivo and In Vitro.Oxid Med Cell Longev. 2022 Sep 1;2022:3800004. doi: 10.1155/2022/3800004. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36092158 Free PMC article.
-
Astrocyte polarization in glaucoma: a new opportunity.Neural Regen Res. 2022 Dec;17(12):2582-2588. doi: 10.4103/1673-5374.339470. Neural Regen Res. 2022. PMID: 35662185 Free PMC article. Review.
Cited by
-
Blocking Two-Pore Domain Potassium Channel TREK-1 Inhibits the Activation of A1-Like Reactive Astrocyte Through the NF-κB Signaling Pathway in a Rat Model of Major Depressive Disorder.Neurochem Res. 2023 Jun;48(6):1737-1754. doi: 10.1007/s11064-023-03857-4. Epub 2023 Jan 20. Neurochem Res. 2023. PMID: 36670238 Free PMC article.
-
MiRNA-338-3p Inhibits Neuroinflammation in the Corpus Callosum of LCV-LPS Rats Via STAT1 Signal Pathway.Cell Mol Neurobiol. 2023 Oct;43(7):3669-3692. doi: 10.1007/s10571-023-01378-w. Epub 2023 Jul 21. Cell Mol Neurobiol. 2023. PMID: 37479855 Free PMC article.
-
The Many Faces of Astrocytes in the Septic Brain.Mol Neurobiol. 2022 Dec;59(12):7229-7235. doi: 10.1007/s12035-022-03027-7. Epub 2022 Sep 22. Mol Neurobiol. 2022. PMID: 36136265 Review.
-
Melatonin: Regulation of Viral Phase Separation and Epitranscriptomics in Post-Acute Sequelae of COVID-19.Int J Mol Sci. 2022 Jul 23;23(15):8122. doi: 10.3390/ijms23158122. Int J Mol Sci. 2022. PMID: 35897696 Free PMC article. Review.
-
Reactive Gliosis in Neonatal Disorders: Friend or Foe for Neuroregeneration?Cells. 2024 Jan 11;13(2):131. doi: 10.3390/cells13020131. Cells. 2024. PMID: 38247822 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

