Outcomes of Patients Who Undergo Transfusion of Fresh Frozen Plasma: A Prospective, Observational, Multicentre Cohort Study in Hiroshima, Japan
- PMID: 34803417
- PMCID: PMC8594890
- DOI: 10.2147/JBM.S338556
Outcomes of Patients Who Undergo Transfusion of Fresh Frozen Plasma: A Prospective, Observational, Multicentre Cohort Study in Hiroshima, Japan
Abstract
Purpose: Given the chronic shortage of blood for transfusion in Japan, promotion of appropriate use of fresh frozen plasma (FFP) urgently needs to be addressed by the national blood project in Japan. Whether FFP transfusions are administered appropriately in Japan is currently unclear. In this study, we aimed to investigate the outcomes of patients who undergo FFP transfusion and the appropriateness of use of FFP.
Patients and methods: This multicentre, prospective, observational cohort study was conducted from September 2017 to April 2019 at the 15 medical institutions in Hiroshima Prefecture that are the top providers of FFP. All patients who underwent FFP transfusion during the study period were included, relevant data being extracted from the medical records. The indications for FFP transfusion were classified in accordance with the Guidelines of the Ministry of Health, Labour and Welfare of Japan. Factors associated with patient outcomes at day 28 after FFP transfusion were subjected to multivariable logistic regression analysis.
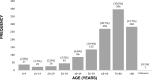
Results: In total, data of 1299 patients were eligible for analysis. At least 63.8% of indications for FFP were in accordance with the guideline for FFP transfusions. The mortality rate at day 28 after FFP transfusion was 16.2%. Older age (65-74 years: adjusted odds ratio [AOR]=4.3, ≥75 years: AOR=4.1), non-perioperative use (AOR=4.5), coagulopathy associated with liver damage (AOR=2.7), large volume of FFP transfused (AOR=2.5), and lack of improvement in blood coagulation following FFP transfusion were independently and significantly associated with death within 28 days after FFP transfusion.
Conclusion: Our findings do not support the simple conclusion that FFP transfusions contribute to prognosis. However, given that coagulopathy in patients with end-stage liver disease is infrequently improved by FFP transfusion, "inappropriate" use of FFP should be avoided. It is important to promote appropriate use of FFP so as not to waste blood resources.
Keywords: coagulopathy; compliance with guideline; inappropriate use; prognosis.
© 2021 Sugiyama et al.
Conflict of interest statement
Dr Masazumi Okajima reports grants from Ministry of Health, Labour and Welfare of Japan, during the conduct of the study; personal fees from Chugai Pharmaceutical Co., Ltd., Taiho Pharma, Johnson and Johnson K. K., Medicaroid Corporation, Eli Lily Japan K. K., Olympus Corporation, and Covidien Japan Inc., outside the submitted work. The authors declare that they have no other conflicts of interest in this work.
Figures
Similar articles
-
Association Between Ratio of Fresh Frozen Plasma to Red Blood Cells During Massive Transfusion and Survival Among Patients Without Traumatic Injury.JAMA Surg. 2017 Jun 1;152(6):574-580. doi: 10.1001/jamasurg.2017.0098. JAMA Surg. 2017. PMID: 28273299 Free PMC article.
-
Fresh frozen plasma transfusion in acute variceal haemorrhage: Results from a multicentre cohort study.Liver Int. 2021 Aug;41(8):1901-1908. doi: 10.1111/liv.14936. Epub 2021 May 24. Liver Int. 2021. PMID: 33969607
-
Retrospective evaluation of the indications, safety and effects of fresh frozen plasma transfusions in 36 cats (2014-2018).J Feline Med Surg. 2020 Aug;22(8):696-704. doi: 10.1177/1098612X19876728. Epub 2019 Oct 2. J Feline Med Surg. 2020. PMID: 31576775 Free PMC article.
-
Perioperative coagulation management--fresh frozen plasma.Best Pract Res Clin Anaesthesiol. 2010 Mar;24(1):51-64. doi: 10.1016/j.bpa.2009.09.007. Best Pract Res Clin Anaesthesiol. 2010. PMID: 20402170 Review.
-
Impact of transfusion of fresh-frozen plasma and packed red blood cells in a 1:1 ratio on survival of emergency department patients with severe trauma.Acad Emerg Med. 2009 May;16(5):371-8. doi: 10.1111/j.1553-2712.2009.00386.x. Epub 2009 Mar 16. Acad Emerg Med. 2009. PMID: 19302364 Review.
Cited by
-
Platelet Function Testing Using Sonoclot and TEG6s as a Platelet Transfusion Prediction Tool in Open Heart Surgery.Cureus. 2023 Nov 20;15(11):e49131. doi: 10.7759/cureus.49131. eCollection 2023 Nov. Cureus. 2023. PMID: 38130528 Free PMC article.
-
Individualized Management of Coagulopathy in Patients with End-Stage Liver Disease.Diagnostics (Basel). 2022 Dec 15;12(12):3172. doi: 10.3390/diagnostics12123172. Diagnostics (Basel). 2022. PMID: 36553179 Free PMC article. Review.
-
Efficacy of fresh frozen plasma transfusion in decompensated cirrhosis patients with coagulopathy admitted to ICU: a retrospective cohort study from MIMIC-IV database.Sci Rep. 2024 Feb 28;14(1):4925. doi: 10.1038/s41598-024-54379-0. Sci Rep. 2024. PMID: 38418492 Free PMC article.
References
-
- Ministry of Health. Labour and welfare of Japan: annual report on national blood project in 2016. Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000154567.html. Accessed December 9, 2020.
-
- Ministry of Health. Labour and welfare of Japan: annual report on national blood project in 2020. Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000063233.html. Accessed August 4, 2021.
-
- Ministry of Health. Labour and welfare of Japan: the guideline for the use of blood products. First edition. Available from: https://www.mhlw.go.jp/new-info/kobetu/iyaku/kenketsugo/5tekisei3b.html. Accessed December 7, 2020.
-
- Ministry of Health. Labour and welfare of Japan: the guideline for the use of blood products (revised in 2017). Available from: https://www.mhlw.go.jp/file/06-Seisakujouhou-11120000-Iyakushokuhinkyoku.... Accessed December 9, 2020.
LinkOut - more resources
Full Text Sources
Miscellaneous