Binary-QSAR guided virtual screening of FDA approved drugs and compounds in clinical investigation against SARS-CoV-2 main protease
- PMID: 34803447
- PMCID: PMC8573836
- DOI: 10.3906/biy-2106-61
Binary-QSAR guided virtual screening of FDA approved drugs and compounds in clinical investigation against SARS-CoV-2 main protease
Abstract
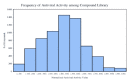
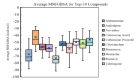
With the emergence of the new SARS-CoV-2 virus, drug repurposing studies have gained substantial importance. Combined with the efficacy of recent improvements in ligand- and target-based virtual screening approaches, virtual screening has become faster and more productive than ever. In the current study, an FDA library of approved drugs and compounds under clinical investigation were screened for their antiviral activity using the antiviral therapeutic activity binary QSAR model of the MetaCore/MetaDrug platform. Among 6733-compound collection, we found 370 compounds with a normalized therapeutic activity value greater than a cutoff of 0.75. Only these selected compounds were used for molecular docking studies against the SARS-CoV-2 main protease (Mpro). After initial short (10 ns) molecular dynamics (MD) simulations with the top-50 docking scored compounds and following molecular mechanics generalized born surface area (MM/GBSA) calculations, top-10 compounds were subjected to longer (100 ns) MD simulations and end-point MM/GBSA estimations. Our virtual screening protocol yielded Cefuroxime pivoxetil, an ester prodrug of second-generation cephalosporin antibiotic Cefuroxime, as being a considerable molecule for drug repurposing against the SARS-CoV-2 Mpro.
Keywords: SARS-CoV-2; drug repurposing; virtual screening; Binary QSAR.
Copyright © 2021 The Author(s).
Conflict of interest statement
CONFLICT OF INTEREST: The authors declare that there is not any conflict of interest.
Figures

Similar articles
-
Virtual drug repurposing study against SARS-CoV-2 TMPRSS2 target.Turk J Biol. 2020 Jun 21;44(3):185-191. doi: 10.3906/biy-2005-112. eCollection 2020. Turk J Biol. 2020. PMID: 32595355 Free PMC article.
-
Screening of FDA approved drugs for finding potential inhibitors against Granzyme B as a potent drug-repurposing target.J Mol Graph Model. 2020 Mar;95:107462. doi: 10.1016/j.jmgm.2019.107462. Epub 2019 Oct 11. J Mol Graph Model. 2020. PMID: 31786094
-
Integrating Ligand and Target-Driven Based Virtual Screening Approaches With in vitro Human Cell Line Models and Time-Resolved Fluorescence Resonance Energy Transfer Assay to Identify Novel Hit Compounds Against BCL-2.Front Chem. 2020 Apr 9;8:167. doi: 10.3389/fchem.2020.00167. eCollection 2020. Front Chem. 2020. PMID: 32328476 Free PMC article.
-
In silico prediction of potential inhibitors for the main protease of SARS-CoV-2 using molecular docking and dynamics simulation based drug-repurposing.J Infect Public Health. 2020 Sep;13(9):1210-1223. doi: 10.1016/j.jiph.2020.06.016. Epub 2020 Jun 16. J Infect Public Health. 2020. PMID: 32561274 Free PMC article.
-
Identification of lead compounds from large natural product library targeting 3C-like protease of SARS-CoV-2 using E-pharmacophore modelling, QSAR and molecular dynamics simulation.In Silico Pharmacol. 2021 Aug 7;9(1):49. doi: 10.1007/s40203-021-00109-7. eCollection 2021. In Silico Pharmacol. 2021. PMID: 34395160 Free PMC article.
Cited by
-
Scope of repurposed drugs against the potential targets of the latest variants of SARS-CoV-2.Struct Chem. 2022;33(5):1585-1608. doi: 10.1007/s11224-022-02020-z. Epub 2022 Aug 3. Struct Chem. 2022. PMID: 35938064 Free PMC article. Review.
References
-
- Akhoon BA Tiwari H Nargotra A In silico drug design methods for drug repurposing. In Silico Drug Design. 2019:47–84.
-
- Dalerba P Levin B Thompson JL A trial of lopinavir-ritonavir in. Covid-19. The New England Journal of Medicine. 2020;382 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
