Systemic Deficiency of GHR in Pigs leads to Hepatic Steatosis via Negative Regulation of AHR Signaling
- PMID: 34803486
- PMCID: PMC8579453
- DOI: 10.7150/ijbs.64894
Systemic Deficiency of GHR in Pigs leads to Hepatic Steatosis via Negative Regulation of AHR Signaling
Abstract
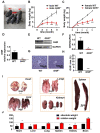
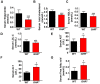
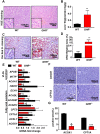
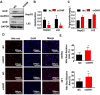
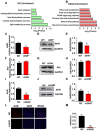
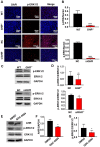
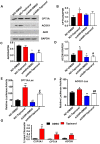
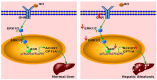
Laron syndrome (LS) is an autosomal recessive genetic disease mainly caused by mutations in the human growth hormone receptor (GHR) gene. Previous studies have focused on Ghr mutant mice, but compared with LS patients, Ghr knockout (KO) mice exhibit differential lipid metabolism. To elucidate the relationship between GHR mutation and lipid metabolism, the role of GHR in lipid metabolism was examined in GHR KO pigs and hepatocytes transfected with siGHR. We observed high levels of free fatty acids and hepatic steatosis in GHR KO pigs, which recapitulates the abnormal lipid metabolism in LS patients. RNAseq analysis revealed that genes related to the fatty acid oxidation pathway were significantly altered in GHR KO pigs. AHR, a transcription factor related to lipid metabolism, was significantly downregulated in GHR KO pigs and siGHR-treated human hepatocytes. We found that AHR directly regulated fatty acid oxidation by directly binding to the promoters of ACOX1 and CPT1A and activating their expression. These data indicate that loss of GHR disturbs the ERK-AHR-ACOX1/CPT1A pathway and consequently leads to hepatic steatosis. Our results established AHR as a modulator of hepatic steatosis, thereby providing a therapeutic target for lipid metabolism disorder.
Keywords: AHR; GHR; Laron syndrome; hepatic steatosis.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Alessia D, Vivian H, Metherell L A, Irène N, Cecilia C H, Clark A. et al. Evidence for a continuum of genetic, phenotypic, and biochemical abnormalities in children with growth hormone insensitivity. Endocr. Rev. 2011;32:472–494. - PubMed
-
- Brooks A J, Wooh J W, Tunny K A, Waters M J. Growth hormone receptor; mechanism of action. Int J Biochem Cell B. 2008;40:1984–1989. - PubMed
-
- Vanderkuur J A, Butch E R, Waters S B, Pessin J E, Kun-Liang G, Christin C S. Signaling molecules involved in coupling growth hormone receptor to mitogen-activated protein kinase activation. Endocrinology. 1997;138:4301–4307. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

