Long term usage of dexamethasone accelerating accelerates the initiation of osteoarthritis via enhancing chondrocyte apoptosis and the extracellular matrix calcification and apoptosis of chondrocytes
- PMID: 34803488
- PMCID: PMC8579451
- DOI: 10.7150/ijbs.64152
Long term usage of dexamethasone accelerating accelerates the initiation of osteoarthritis via enhancing chondrocyte apoptosis and the extracellular matrix calcification and apoptosis of chondrocytes
Abstract
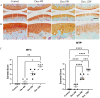
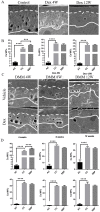
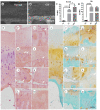
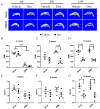
Systemic application of glucocorticoids is an essential anti-inflammatory and immune-modulating therapy for severe inflammatory or autoimmunity conditions. However, its long-term effects on articular cartilage of patients' health need to be further investigated. In this study, we studied the effects of dexamethasone (Dex) on the homeostasis of articular cartilage and the progress of destabilization of medial meniscus (DMM)-induced osteoarthritis (OA) in adult mice. Long-term administration of Dex aggravates the proteoglycan loss of articular cartilage and drastically accelerates cartilage degeneration under surgically induced OA conditions. In addition, Dex increases calcium content in calcified cartilage layer of mice and the samples from OA patients with a history of long-term Dex treatment. Moreover, long term usage of Dex results in decrease subchondral bone mass and bone density. Further studies showed that Dex leads to calcification of extracellular matrix of chondrocytes partially through activation of AKT, as well as promotes apoptosis of chondrocytes in calcified cartilage layer. Besides, Dex weakens the stress-response autophagy with the passage of time. Taken together, our data indicate that long-term application of Dex may predispose patients to OA and or even accelerate the OA disease progression development of OA patients.
Keywords: AKT; apoptosis; articular cartilage; calcification; dexamethasone.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
A novel FGFR1-binding peptide attenuates the degeneration of articular cartilage in adult mice.Osteoarthritis Cartilage. 2018 Dec;26(12):1733-1743. doi: 10.1016/j.joca.2018.08.012. Epub 2018 Sep 8. Osteoarthritis Cartilage. 2018. PMID: 30201491
-
High concentration magnesium inhibits extracellular matrix calcification and protects articular cartilage via Erk/autophagy pathway.J Cell Physiol. 2019 Dec;234(12):23190-23201. doi: 10.1002/jcp.28885. Epub 2019 Jun 3. J Cell Physiol. 2019. PMID: 31161622
-
Lactoferrin inhibits dexamethasone-induced chondrocyte impairment from osteoarthritic cartilage through up-regulation of extracellular signal-regulated kinase 1/2 and suppression of FASL, FAS, and Caspase 3.Biochem Biophys Res Commun. 2013 Nov 8;441(1):249-55. doi: 10.1016/j.bbrc.2013.10.047. Epub 2013 Oct 17. Biochem Biophys Res Commun. 2013. PMID: 24141118
-
Chondrocyte Apoptosis in the Pathogenesis of Osteoarthritis.Int J Mol Sci. 2015 Oct 30;16(11):26035-54. doi: 10.3390/ijms161125943. Int J Mol Sci. 2015. PMID: 26528972 Free PMC article. Review.
-
Molecular regulation of articular chondrocyte function and its significance in osteoarthritis.Histol Histopathol. 2011 Mar;26(3):377-94. doi: 10.14670/HH-26.377. Histol Histopathol. 2011. PMID: 21210351 Review.
Cited by
-
Knowledge mapping of autophagy in osteoarthritis from 2004 to 2022: A bibliometric analysis.Front Immunol. 2023 Mar 9;14:1063018. doi: 10.3389/fimmu.2023.1063018. eCollection 2023. Front Immunol. 2023. PMID: 36969240 Free PMC article.
-
Dexamethasone: a double-edged sword in the treatment of osteoarthritis.Sci Rep. 2025 Apr 7;15(1):11832. doi: 10.1038/s41598-025-96050-2. Sci Rep. 2025. PMID: 40195473 Free PMC article.
-
Ajugol's upregulation of TFEB-mediated autophagy alleviates endoplasmic reticulum stress in chondrocytes and retards osteoarthritis progression in a mouse model.Chin Med. 2023 Sep 7;18(1):113. doi: 10.1186/s13020-023-00824-7. Chin Med. 2023. PMID: 37679844 Free PMC article.
-
Targeted and responsive biomaterials in osteoarthritis.Theranostics. 2023 Jan 16;13(3):931-954. doi: 10.7150/thno.78639. eCollection 2023. Theranostics. 2023. PMID: 36793867 Free PMC article. Review.
-
Osteoarthritis: pathogenic signaling pathways and therapeutic targets.Signal Transduct Target Ther. 2023 Feb 3;8(1):56. doi: 10.1038/s41392-023-01330-w. Signal Transduct Target Ther. 2023. PMID: 36737426 Free PMC article. Review.
References
-
- Yoon BH, Jones LC, Chen CH, Cheng EY, Cui Q, Drescher W. et al. Etiologic Classification Criteria of ARCO on Femoral Head Osteonecrosis Part 1: Glucocorticoid-Associated Osteonecrosis. The Journal of arthroplasty. 2019;34:163–8.e1. - PubMed
-
- McDonough AK, Curtis JR, Saag KG. The epidemiology of glucocorticoid-associated adverse events. Current opinion in rheumatology. 2008;20:131–7. - PubMed
-
- van Staa TP, Leufkens HG, Abenhaim L, Begaud B, Zhang B, Cooper C. Use of oral corticosteroids in the United Kingdom. QJM: monthly journal of the Association of Physicians. 2000;93:105–11. - PubMed
-
- Cooper C, Steinbuch M, Stevenson R, Miday R, Watts NB. The epidemiology of osteonecrosis: findings from the GPRD and THIN databases in the UK. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2010;21:569–77. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

