Lipid-induced S-palmitoylation as a Vital Regulator of Cell Signaling and Disease Development
- PMID: 34803494
- PMCID: PMC8579454
- DOI: 10.7150/ijbs.64046
Lipid-induced S-palmitoylation as a Vital Regulator of Cell Signaling and Disease Development
Abstract
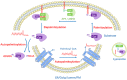
Lipid metabolites are emerging as pivotal regulators of protein function and cell signaling. The availability of intracellular fatty acid is tightly regulated by glycolipid metabolism and may affect human body through many biological mechanisms. Recent studies have demonstrated palmitate, either from exogenous fatty acid uptake or de novo fatty acid synthesis, may serve as the substrate for protein palmitoylation and regulate protein function via palmitoylation. Palmitoylation, the most-studied protein lipidation, encompasses the reversible covalent attachment of palmitate moieties to protein cysteine residues. It controls various cellular physiological processes and alters protein stability, conformation, localization, membrane association and interaction with other effectors. Dysregulation of palmitoylation has been implicated in a plethora of diseases, such as metabolic syndrome, cancers, neurological disorders and infections. Accordingly, it could be one of the molecular mechanisms underlying the impact of palmitate metabolite on cellular homeostasis and human diseases. Herein, we explore the relationship between lipid metabolites and the regulation of protein function through palmitoylation. We review the current progress made on the putative role of palmitate in altering the palmitoylation of key proteins and thus contributing to the pathogenesis of various diseases, among which we focus on metabolic disorders, cancers, inflammation and infections, neurodegenerative diseases. We also highlight the opportunities and new therapeutics to target palmitoylation in disease development.
Keywords: Cancer; Inflammation; Lipid metabolism; Neurodegeneration; Palmitoylation.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
Putative Role of Protein Palmitoylation in Cardiac Lipid-Induced Insulin Resistance.Int J Mol Sci. 2020 Dec 11;21(24):9438. doi: 10.3390/ijms21249438. Int J Mol Sci. 2020. PMID: 33322406 Free PMC article. Review.
-
Protein Lipidation by Palmitate Controls Macrophage Function.Cells. 2022 Feb 6;11(3):565. doi: 10.3390/cells11030565. Cells. 2022. PMID: 35159374 Free PMC article. Review.
-
Protein Lipidation by Palmitoylation and Myristoylation in Cancer.Front Cell Dev Biol. 2021 May 20;9:673647. doi: 10.3389/fcell.2021.673647. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34095144 Free PMC article. Review.
-
Protein Lipidation in Cell Signaling and Diseases: Function, Regulation, and Therapeutic Opportunities.Cell Chem Biol. 2018 Jul 19;25(7):817-831. doi: 10.1016/j.chembiol.2018.05.003. Epub 2018 May 31. Cell Chem Biol. 2018. PMID: 29861273 Free PMC article. Review.
-
Protein S-palmitoylation in cellular differentiation.Biochem Soc Trans. 2017 Feb 8;45(1):275-285. doi: 10.1042/BST20160236. Biochem Soc Trans. 2017. PMID: 28202682 Free PMC article. Review.
Cited by
-
Proteomic analysis of s-acylated proteins in human retinal pigment epithelial cells and the role of palmitoylation of Niemann-Pick type C1 protein in cholesterol transport.Front Aging Neurosci. 2022 Oct 3;14:965943. doi: 10.3389/fnagi.2022.965943. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36262888 Free PMC article.
-
Protein palmitoylation: biological functions, disease, and therapeutic targets.MedComm (2020). 2025 Feb 21;6(3):e70096. doi: 10.1002/mco2.70096. eCollection 2025 Mar. MedComm (2020). 2025. PMID: 39991624 Free PMC article. Review.
-
CLDN6 inhibits breast cancer growth and metastasis through SREBP1-mediated RAS palmitoylation.Cell Mol Biol Lett. 2024 Aug 21;29(1):112. doi: 10.1186/s11658-024-00629-y. Cell Mol Biol Lett. 2024. PMID: 39169280 Free PMC article.
-
Post-translational palmitoylation of metabolic proteins.Front Physiol. 2023 Feb 24;14:1122895. doi: 10.3389/fphys.2023.1122895. eCollection 2023. Front Physiol. 2023. PMID: 36909239 Free PMC article. Review.
-
Spatiotemporal control of kinases and the biomolecular tools to trace activity.J Biol Chem. 2024 Nov;300(11):107846. doi: 10.1016/j.jbc.2024.107846. Epub 2024 Oct 1. J Biol Chem. 2024. PMID: 39362469 Free PMC article. Review.
References
-
- Ziegler AB, Thiele C, Tenedini F. et al. Cell-Autonomous Control of Neuronal Dendrite Expansion via the Fatty Acid Synthesis Regulator SREBP. Cell Rep. 2017;21:3346–3353. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

