Laboratory Monitoring of Mother, Fetus, and Newborn in Hemolytic Disease of Fetus and Newborn
- PMID: 34803574
- PMCID: PMC8578801
- DOI: 10.1159/000518782
Laboratory Monitoring of Mother, Fetus, and Newborn in Hemolytic Disease of Fetus and Newborn
Abstract
Background: Laboratory monitoring of mother, fetus, and newborn in hemolytic disease of fetus and newborn (HDFN) aims to guide clinicians and the immunized women to focus on the most serious problems of alloimmunization and thus minimize the consequences of HDFN in general and of anti-D in particular. Here, we present the current approach of laboratory screening and testing for prevention and monitoring of HDFN at the Copenhagen University Hospital in Denmark.
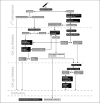
Summary: All pregnant women are typed and screened in the 1st trimester. This serves to identify the RhD-negative pregnant women who at gestational age (GA) of 25 weeks are offered a second screen test and a non-invasive fetal RhD prediction. At GA 29 weeks, and again after delivery, non-immunized RhD-negative women carrying an RhD-positive fetus are offered Rh immunoglobulin. If the 1st trimester screen reveals an alloantibody, antenatal investigation is initiated. This also includes RhD-positive women with alloantibodies. Specificity and titer are determined, the fetal phenotype is predicted by non-invasive genotyping based on cell-free DNA (RhD, K, Rhc, RhC, RhE, ABO), and serial monitoring of titer commences. Based on titers and specificity, monitoring with serial peak systolic velocity measurements in the fetal middle cerebral artery to detect anemia will take place. Intrauterine transfusion is given when fetal anemia is suspected. Monitoring of the newborn by titer and survival of fetal red blood cells by flow cytometry will help predict the length of the recovery of the newborn.
Keywords: Alloimmunization; Cell-free DNA; Hemolytic disease of fetus and newborn; Middle cerebral artery, Peak systolic velocity; Next-generation sequencing.
Copyright © 2021 by S. Karger AG, Basel.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Bowman JM, Pollock JM, Penston LE. Fetomaternal transplacental hemorrhage during pregnancy and after delivery. Vox Sang. 1986;51((2)):117–21. - PubMed
-
- de Haas M, Thurik FF, Koelewijn JM, van der Schoot CE. Haemolytic disease of the fetus and newborn. Vox Sang. 2015 Aug;109((2)):99–113. - PubMed
-
- Koelewijn JM, Vrijkotte TG, de Haas M, van der Schoot CE, Bonsel GJ. Risk factors for the presence of non-rhesus D red blood cell antibodies in pregnancy. BJOG. 2009 Apr;116((5)):655–64. - PubMed
-
- Firan M, Bawdon R, Radu C, Ober RJ, Eaken D, Antohe F, et al. The MHC class I-related receptor, FcRn, plays an essential role in the maternofetal transfer of gamma-globulin in humans. Int Immunol. 2001 Aug;13((8)):993–1002. - PubMed
-
- Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature. 1989 Jan 12;337((6203)):184–7. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

