Dysregulation of Neuronal Nicotinic Acetylcholine Receptor-Cholesterol Crosstalk in Autism Spectrum Disorder
- PMID: 34803605
- PMCID: PMC8604044
- DOI: 10.3389/fnmol.2021.744597
Dysregulation of Neuronal Nicotinic Acetylcholine Receptor-Cholesterol Crosstalk in Autism Spectrum Disorder
Abstract
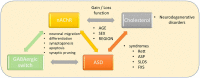
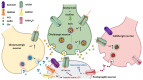
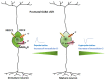
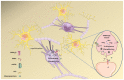
Autism spectrum disorder (ASD) is a set of complex neurodevelopmental diseases that include impaired social interaction, delayed and disordered language, repetitive or stereotypic behavior, restricted range of interests, and altered sensory processing. The underlying causes of the core symptoms remain unclear, as are the factors that trigger their onset. Given the complexity and heterogeneity of the clinical phenotypes, a constellation of genetic, epigenetic, environmental, and immunological factors may be involved. The lack of appropriate biomarkers for the evaluation of neurodevelopmental disorders makes it difficult to assess the contribution of early alterations in neurochemical processes and neuroanatomical and neurodevelopmental factors to ASD. Abnormalities in the cholinergic system in various regions of the brain and cerebellum are observed in ASD, and recently altered cholesterol metabolism has been implicated at the initial stages of the disease. Given the multiple effects of the neutral lipid cholesterol on the paradigm rapid ligand-gated ion channel, the nicotinic acetylcholine receptor, we explore in this review the possibility that the dysregulation of nicotinic receptor-cholesterol crosstalk plays a role in some of the neurological alterations observed in ASD.
Keywords: acetylcholine receptor-cholesterol interactions; autism spectrum disorder; cholesterol; nicotinic receptor (nAChR); perinatal period.
Copyright © 2021 Vallés and Barrantes.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Amadeo A., Coatti A., Aracri P., Ascagni M., Iannantuoni D., Modena D., et al. (2018). Postnatal changes in K + /Cl − cotransporter-2 expression in the forebrain of mice bearing a mutant nicotinic subunit linked to sleep-related epilepsy. Neuroscience 386, 91–107. 10.1016/j.neuroscience.2018.06.030 - DOI - PubMed
LinkOut - more resources
Full Text Sources

