Circulating Natural Autoantibodies to HER2-Derived Peptides Performed Antitumor Effects on Oral Squamous Cell Carcinoma
- PMID: 34803666
- PMCID: PMC8602057
- DOI: 10.3389/fphar.2021.693989
Circulating Natural Autoantibodies to HER2-Derived Peptides Performed Antitumor Effects on Oral Squamous Cell Carcinoma
Abstract
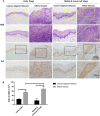
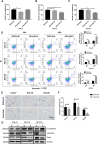
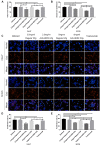
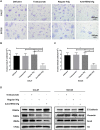
Natural autoantibodies play a crucial role in destruction of malignant tumors due to immune surveillance function. Epidermal growth factor receptor 2 (HER2) has been found to be highly expressed in a variety of epithelial tumors including oral squamous cell carcinoma (OSCC). The present study was thus undertaken to investigate the effect of anti-HER2 natural autoantibodies on OSCC. Compared with cancer-adjacent tissues, cancer tissues from OSCC patients exhibited higher HER2 expression especially in those with middle & advanced stage OSCC. Plasma anti-HER2 IgG levels examined with an enzyme-linked immunosorbent assay (ELISA) developed in-house showed differences between control subjects, individuals with oral benign tumor and patients with OSCC. In addition, anti-HER2 IgG-abundant plasma was screened from healthy donors to treat OSCC cells and to prepare for anti-HER2 intravenous immunoglobulin (IVIg). Both anti-HER2 IgG-abundant plasma and anti-HER2 IVIg could significantly inhibit proliferation and invasion of OSCC cells by inducing the apoptosis, and also regulate apoptosis-associated factors and epithelial-mesenchymal transition (EMT), respectively. Besides, the complement-dependent cytotoxicity (CDC) pathway was likely to contribute to the anti-HER2 IgG mediated inhibition of OSCC cells. After the HER2 gene was knocked down with HER2-specific siRNAs, the inhibitory effects on OSCC cell proliferation and apoptotic induction faded away. In conclusion, human plasma IgG, or IVIg against HER2 may be a promising agent for anti-OSCC therapy.
Keywords: HER2; circulating natural autoantibodies; intravenous immunoglobulin; oral squamous cell carcinoma; trastuzumab.
Copyright © 2021 Liu, He, Qu, Meng, Qin and Hu.
Conflict of interest statement
Hailanshen Biotechnology Ltd., Qingdao, China provided all the ELISA reagents for free of charge. Boya Bio-pharmaceutical Group Co., Ltd., China kindly provided anti-HER2 IVIg for free of charge.
Figures



References
-
- Alonso W., Vandeberg P., Lang J., Yuziuk J., Silverstein R., Stokes K., et al. (2020). Immune Globulin Subcutaneous, Human 20% Solution (Xembify®), a New High Concentration Immunoglobulin Product for Subcutaneous Administration. Biologicals 64, 34–40. 10.1016/j.biologicals.2020.01.004 - DOI - PubMed
-
- Barthélémy P., Leblanc J., Goldbarg V., Wendling F., Kurtz J. E. (2014). Pertuzumab: Development beyond Breast Cancer. Anticancer Res. 34 (4), 1483–1491. - PubMed
-
- Cameron D., Piccart-Gebhart M. J., Gelber R. D., Procter M., Goldhirsch A., de Azambuja E., et al. (2017). 11 Years' Follow-Up of Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Early Breast Cancer: Final Analysis of the HERceptin Adjuvant (HERA) Trial. Lancet 389 (10075), 1195–1205. 10.1016/s0140-6736(16)32616-2 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

