Transgenic Overexpression of Galectin-3 in Pancreatic β Cells Attenuates Hyperglycemia in Mice: Synergistic Antidiabetic Effect With Exogenous IL-33
- PMID: 34803672
- PMCID: PMC8602837
- DOI: 10.3389/fphar.2021.714683
Transgenic Overexpression of Galectin-3 in Pancreatic β Cells Attenuates Hyperglycemia in Mice: Synergistic Antidiabetic Effect With Exogenous IL-33
Abstract
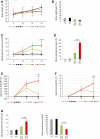
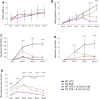
Galectin-3 (Gal-3) has diverse roles in inflammatory and autoimmune diseases. There is evidence that Gal-3 plays a role in both type 1 and type 2 diabetes. While the role of Gal-3 expression in immune cells invading the pancreatic islets in the experimental model of type 1 diabetes mellitus has been already studied, the importance of the overexpression of Gal-3 in the target β cells is not defined. Therefore, we used multiple low doses of streptozotocin (MLD-STZ)-induced diabetes in C57Bl/6 mice to analyze the effect of transgenic (TG) overexpression of Gal-3 in β cells. Our results demonstrated that the overexpression of Gal-3 protected β cells from apoptosis and attenuated MLD-STZ-induced hyperglycemia, glycosuria, and ketonuria. The cellular analysis of pancreata and draining lymph nodes showed that Gal-3 overexpression significantly decreased the number of pro-inflammatory cells without affecting the presence of T-regulatory cells. As the application of exogenous interleukin 33 (IL-33) given from the beginning of MLD-STZ diabetes induction attenuates the development of disease, by increasing the presence of regulatory FoxP3+ ST2+ cells, we evaluated the potential synergistic effect of the exogenous IL-33 and TG overexpression of Gal-3 in β cells at the later stage of diabetogenesis. The addition of IL-33 potentiated the survival of β cells and attenuated diabetes even when administered later, after the onset of hyperglycemia (12-18 days), suggesting that protection from apoptosis and immunoregulation by IL-33 may attenuate type 1 diabetes.
Keywords: galectin-3; interleukin 33 (IL-33); regulatory T cells (T reg); type 1 diabetes mellitus (T1D); β cells.
Copyright © 2021 Jovicic, Petrovic, Pejnovic, Ljujic, Miletic Kovacevic, Pavlovic, Jeftic, Djukic, Srejovic, Jakovljevic and Lukic.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Akahani S., Nangia-Makker P., Inohara H., Kim H. R., Raz A. (1997). Galectin-3: a Novel Anti-apoptotic Molecule with a Functional BH1 (NWGR) Domain of Bcl-2 Family. Cancer Res. 57, 5272–5276. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

