A Comprehensive Account on Recent Progress in Pharmacological Activities of Benzimidazole Derivatives
- PMID: 34803707
- PMCID: PMC8597275
- DOI: 10.3389/fphar.2021.762807
A Comprehensive Account on Recent Progress in Pharmacological Activities of Benzimidazole Derivatives
Abstract
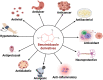
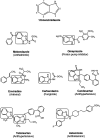
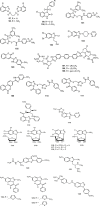
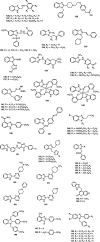
Nowadays, nitrogenous heterocyclic molecules have attracted a great deal of interest among medicinal chemists. Among these potential heterocyclic drugs, benzimidazole scaffolds are considerably prevalent. Due to their isostructural pharmacophore of naturally occurring active biomolecules, benzimidazole derivatives have significant importance as chemotherapeutic agents in diverse clinical conditions. Researchers have synthesized plenty of benzimidazole derivatives in the last decades, amidst a large share of these compounds exerted excellent bioactivity against many ailments with outstanding bioavailability, safety, and stability profiles. In this comprehensive review, we have summarized the bioactivity of the benzimidazole derivatives reported in recent literature (2012-2021) with their available structure-activity relationship. Compounds bearing benzimidazole nucleus possess broad-spectrum pharmacological properties ranging from common antibacterial effects to the world's most virulent diseases. Several promising therapeutic candidates are undergoing human trials, and some of these are going to be approved for clinical use. However, notable challenges, such as drug resistance, costly and tedious synthetic methods, little structural information of receptors, lack of advanced software, and so on, are still viable to be overcome for further research.
Keywords: anti-infectious; anti-proliferative; cardiovascular agents; diverse pharmacological activities; nitrogenous heterocyclic compounds; structure-activity relationship (SAR).
Copyright © 2021 Brishty, Hossain, Khandaker, Faruque, Osman and Rahman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



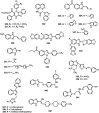
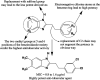
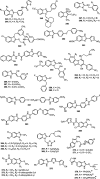
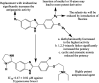
Similar articles
-
Unlocking the Pharmacological Potential of Benzimidazole Derivatives: A Pathway to Drug Development.Curr Top Med Chem. 2024;24(5):437-485. doi: 10.2174/0115680266283641240109080047. Curr Top Med Chem. 2024. PMID: 38311918 Review.
-
An Outline on Benzimidazole Containing Marketed Drugs with Proton Pump Inhibitor and H1 Receptor Antagonist Activities.Mini Rev Med Chem. 2025;25(6):440-462. doi: 10.2174/0113895575329633240928163509. Mini Rev Med Chem. 2025. PMID: 39779557 Review.
-
In silico Approaches for Exploring the Pharmacological Activities of Benzimidazole Derivatives: A Comprehensive Review.Mini Rev Med Chem. 2024;24(16):1481-1495. doi: 10.2174/0113895575287322240115115125. Mini Rev Med Chem. 2024. PMID: 38288816 Review.
-
Recent Developments in the Antimicrobial Potential of Some Nitrogenous Heterocycles and their SAR Studies: A Review.Curr Med Chem. 2024 May 23. doi: 10.2174/0109298673301266240506083014. Online ahead of print. Curr Med Chem. 2024. PMID: 38797910
-
New substituted benzimidazole derivatives: a patent review (2010 - 2012).Expert Opin Ther Pat. 2013 Sep;23(9):1157-79. doi: 10.1517/13543776.2013.800857. Epub 2013 May 16. Expert Opin Ther Pat. 2013. PMID: 23675911 Review.
Cited by
-
Design and synthesis of novel coumarin-benzimidazole hybrids as human galectin-1 inhibitors.Future Med Chem. 2024;16(9):843-857. doi: 10.4155/fmc-2023-0273. Epub 2024 Apr 12. Future Med Chem. 2024. PMID: 38606540 Free PMC article.
-
Chemometric modeling, inverse docking, and molecular simulations-driven design for multilayered prioritization of novel leishmanicidal agents based on a 2-aminobenzimidazole scaffold.Mol Divers. 2025 Jun 16. doi: 10.1007/s11030-025-11228-0. Online ahead of print. Mol Divers. 2025. PMID: 40524132
-
Unlocking the Pharmacological Potential of Benzimidazole Derivatives: A Pathway to Drug Development.Curr Top Med Chem. 2024;24(5):437-485. doi: 10.2174/0115680266283641240109080047. Curr Top Med Chem. 2024. PMID: 38311918 Review.
-
Benzimidazole-Triazole Hybrids as Antimicrobial and Antiviral Agents: A Systematic Review.Antibiotics (Basel). 2023 Jul 22;12(7):1220. doi: 10.3390/antibiotics12071220. Antibiotics (Basel). 2023. PMID: 37508316 Free PMC article. Review.
-
From Deworming to Cancer Therapy: Benzimidazoles in Hematological Malignancies.Cancers (Basel). 2024 Oct 12;16(20):3454. doi: 10.3390/cancers16203454. Cancers (Basel). 2024. PMID: 39456548 Free PMC article. Review.
References
-
- Abdelgawad M. A., Bakr R. B., Ahmad W., Al-Sanea M. M., Elshemy H. A. H. (2019). New Pyrimidine-Benzoxazole/benzimidazole Hybrids: Synthesis, Antioxidant, Cytotoxic Activity, In Vitro Cyclooxygenase and Phospholipase A2-V Inhibition. Bioorg. Chem. 92, 103218. 10.1016/j.bioorg.2019.103218 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

