Evaluation of Kratom Opioid Derivatives as Potential Treatment Option for Alcohol Use Disorder
- PMID: 34803709
- PMCID: PMC8596301
- DOI: 10.3389/fphar.2021.764885
Evaluation of Kratom Opioid Derivatives as Potential Treatment Option for Alcohol Use Disorder
Abstract
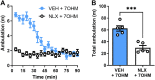
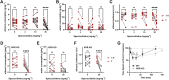
Background and Purpose: Mitragyna speciosa extract and kratom alkaloids decrease alcohol consumption in mice at least in part through actions at the δ-opioid receptor (δOR). However, the most potent opioidergic kratom alkaloid, 7-hydroxymitragynine, exhibits rewarding properties and hyperlocomotion presumably due to preferred affinity for the mu opioid receptor (µOR). We hypothesized that opioidergic kratom alkaloids like paynantheine and speciogynine with reduced µOR potency could provide a starting point for developing opioids with an improved therapeutic window to treat alcohol use disorder. Experimental Approach: We characterized paynantheine, speciociliatine, and four novel kratom-derived analogs for their ability to bind and activate δOR, µOR, and κOR. Select opioids were assessed in behavioral assays in male C57BL/6N WT and δOR knockout mice. Key Results: Paynantheine (10 mg∙kg-1, i.p.) produced aversion in a limited conditioned place preference (CPP) paradigm but did not produce CPP with additional conditioning sessions. Paynantheine did not produce robust antinociception but did block morphine-induced antinociception and hyperlocomotion. Yet, at 10 and 30 mg∙kg-1 doses (i.p.), paynantheine did not counteract morphine CPP. 7-hydroxypaynantheine and 7-hydroxyspeciogynine displayed potency at δOR but limited µOR potency relative to 7-hydroxymitragynine in vitro, and dose-dependently decreased voluntary alcohol consumption in WT but not δOR in KO mice. 7-hydroxyspeciogynine has a maximally tolerated dose of at least 10 mg∙kg-1 (s.c.) at which it did not produce significant CPP neither alter general locomotion nor induce noticeable seizures. Conclusion and Implications: Derivatizing kratom alkaloids with the goal of enhancing δOR potency and reducing off-target effects could provide a pathway to develop novel lead compounds to treat alcohol use disorder with an improved therapeutic window.
Keywords: alcohol use disorder; biased signaling; delta opioid receptor; kratom; nociception; reward; seizures.
Copyright © 2021 Gutridge, Chakraborty, Varga, Rhoda, French, Blaine, Royer, Cui, Yuan, Cassell, Szabó, Majumdar and van Rijn.
Conflict of interest statement
Author MS was employed by XiMo Hungary Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
G protein-biased kratom-alkaloids and synthetic carfentanil-amide opioids as potential treatments for alcohol use disorder.Br J Pharmacol. 2020 Apr;177(7):1497-1513. doi: 10.1111/bph.14913. Epub 2020 Jan 24. Br J Pharmacol. 2020. PMID: 31705528 Free PMC article.
-
Pharmacokinetics of Eleven Kratom Alkaloids Following an Oral Dose of Either Traditional or Commercial Kratom Products in Rats.J Nat Prod. 2021 Apr 23;84(4):1104-1112. doi: 10.1021/acs.jnatprod.0c01163. Epub 2021 Feb 23. J Nat Prod. 2021. PMID: 33620222 Free PMC article.
-
Simultaneous quantification of ten key Kratom alkaloids in Mitragyna speciosa leaf extracts and commercial products by ultra-performance liquid chromatography-tandem mass spectrometry.Drug Test Anal. 2019 Aug;11(8):1162-1171. doi: 10.1002/dta.2604. Epub 2019 May 15. Drug Test Anal. 2019. PMID: 30997725 Free PMC article.
-
Kratom Alkaloids for the Treatment of Alcohol Use Disorder.ACS Chem Neurosci. 2024 Dec 18;15(24):4352-4359. doi: 10.1021/acschemneuro.4c00675. Epub 2024 Nov 29. ACS Chem Neurosci. 2024. PMID: 39611792 Review.
-
The medicinal chemistry and neuropharmacology of kratom: A preliminary discussion of a promising medicinal plant and analysis of its potential for abuse.Neuropharmacology. 2018 May 15;134(Pt A):108-120. doi: 10.1016/j.neuropharm.2017.08.026. Epub 2017 Aug 19. Neuropharmacology. 2018. PMID: 28830758 Review.
Cited by
-
Opportunities and Challenges for In Silico Drug Discovery at Delta Opioid Receptors.Pharmaceuticals (Basel). 2022 Jul 15;15(7):873. doi: 10.3390/ph15070873. Pharmaceuticals (Basel). 2022. PMID: 35890173 Free PMC article. Review.
-
Kratom's Emergence and Persistence Within the US Polydrug Epidemic.Curr Addict Rep. 2023;10(2):262-271. doi: 10.1007/s40429-023-00476-5. Epub 2023 Apr 18. Curr Addict Rep. 2023. PMID: 37266191 Free PMC article. Review.
-
Kratom as a potential substance use disorder harm reduction agent.Front Public Health. 2024 May 30;12:1416689. doi: 10.3389/fpubh.2024.1416689. eCollection 2024. Front Public Health. 2024. PMID: 38873312 Free PMC article. Review.
-
Clinical Implications of Kratom (Mitragyna speciosa) Use: a Literature Review.Curr Addict Rep. 2023;10(2):317-334. doi: 10.1007/s40429-023-00478-3. Epub 2023 May 12. Curr Addict Rep. 2023. PMID: 37266188 Free PMC article. Review.
-
An In Vitro Examination of Whether Kratom Extracts Enhance the Cytotoxicity of Low-Dose Doxorubicin against A549 Human Lung Cancer Cells.Molecules. 2024 Mar 21;29(6):1404. doi: 10.3390/molecules29061404. Molecules. 2024. PMID: 38543040 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

