Differential Effects of 'Vaping' on Lipid and Glucose Profiles and Liver Metabolic Markers in Obese Versus Non-obese Mice
- PMID: 34803738
- PMCID: PMC8599937
- DOI: 10.3389/fphys.2021.755124
Differential Effects of 'Vaping' on Lipid and Glucose Profiles and Liver Metabolic Markers in Obese Versus Non-obese Mice
Abstract
Tobacco smoking increases the risk of metabolic disorders due to the combination of harmful chemicals, whereas pure nicotine can improve glucose tolerance. E-cigarette vapour contains nicotine and some of the harmful chemicals found in cigarette smoke at lower levels. To investigate how e-vapour affects metabolic profiles, male Balb/c mice were exposed to a high-fat diet (HFD, 43% fat, 20kJ/g) for 16weeks, and e-vapour in the last 6weeks. HFD alone doubled fat mass and caused dyslipidaemia and glucose intolerance. E-vapour reduced fat mass in HFD-fed mice; only nicotine-containing e-vapour improved glucose tolerance. In chow-fed mice, e-vapour increased lipid content in both blood and liver. Changes in liver metabolic markers may be adaptive responses rather than causal. Future studies can investigate how e-vapour differentially affects metabolic profiles with different diets.
Keywords: abdominal obesity; free fatty acid; glucose tolerance; liver; triglycerides.
Copyright © 2021 Chen, Li, Chan, Zhang, Gorrell, Pollock, Saad and Oliver.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
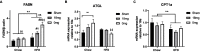

References
-
- Chen H., Ng J. P. M., Bishop D. P., Milthorpe B. K., Valenzuela S. M. (2018b). Gold nanoparticles as cell regulators: beneficial effects of gold nanoparticles on the metabolic profile of mice with pre-existing obesity. J. Nanobiotechnol. 16:88. doi: 10.1186/s12951-018-0414-6, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

