Methods to Develop an in silico Clinical Trial: Computational Head-to-Head Comparison of Lisdexamfetamine and Methylphenidate
- PMID: 34803764
- PMCID: PMC8595241
- DOI: 10.3389/fpsyt.2021.741170
Methods to Develop an in silico Clinical Trial: Computational Head-to-Head Comparison of Lisdexamfetamine and Methylphenidate
Abstract
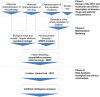
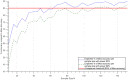
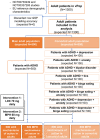
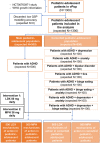
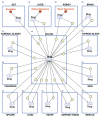
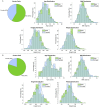
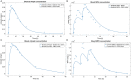
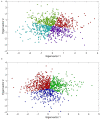
Regulatory agencies encourage computer modeling and simulation to reduce the time and cost of clinical trials. Although still not classified in formal guidelines, system biology-based models represent a powerful tool for generating hypotheses with great molecular detail. Herein, we have applied a mechanistic head-to-head in silico clinical trial (ISCT) between two treatments for attention-deficit/hyperactivity disorder, to wit lisdexamfetamine (LDX) and methylphenidate (MPH). The ISCT was generated through three phases comprising (i) the molecular characterization of drugs and pathologies, (ii) the generation of adult and children virtual populations (vPOPs) totaling 2,600 individuals and the creation of physiologically based pharmacokinetic (PBPK) and quantitative systems pharmacology (QSP) models, and (iii) data analysis with artificial intelligence methods. The characteristics of our vPOPs were in close agreement with real reference populations extracted from clinical trials, as did our PBPK models with in vivo parameters. The mechanisms of action of LDX and MPH were obtained from QSP models combining PBPK modeling of dosing schemes and systems biology-based modeling technology, i.e., therapeutic performance mapping system. The step-by-step process described here to undertake a head-to-head ISCT would allow obtaining mechanistic conclusions that could be extrapolated or used for predictions to a certain extent at the clinical level. Altogether, these computational techniques are proven an excellent tool for hypothesis-generation and would help reach a personalized medicine.
Keywords: attention-deficit/hyperactivity disorder; in silico clinical trial; lisdexamfetamine; mathematical modeling; methylphenidate.
Copyright © 2021 Gutiérrez-Casares, Quintero, Jorba, Junet, Martínez, Pozo-Rubio, Oliva, Daura, Mas and Montoto.
Conflict of interest statement
JRG-C has served as speaker for Takeda and Shire and has received research funding from Shire. JQ has served as speaker and/or on scientific advisory boards for Takeda, Janssen, and Rubio. GJ, VJ, and JM are full-time employees at Anaxomics Biotech. VM, TP-R, and CM are full-time employees at Takeda. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
In silico clinical trial evaluating lisdexamfetamine's and methylphenidate's mechanism of action computational models in an attention-deficit/hyperactivity disorder virtual patients' population.Front Psychiatry. 2023 Jun 2;14:939650. doi: 10.3389/fpsyt.2023.939650. eCollection 2023. Front Psychiatry. 2023. PMID: 37333910 Free PMC article.
-
Randomized, Double-Blind, Placebo-Controlled Acute Comparator Trials of Lisdexamfetamine and Extended-Release Methylphenidate in Adolescents With Attention-Deficit/Hyperactivity Disorder.CNS Drugs. 2017 Nov;31(11):999-1014. doi: 10.1007/s40263-017-0468-2. CNS Drugs. 2017. PMID: 28980198 Free PMC article.
-
Evaluation of a head-to-head study of lisdexamfetamine dimesylate and atomoxetine: evaluation of Dittmann RW, Cardo E, Nagy P, et al. Efficacy and safety of lisdexamfetamine dimesylate and atomoxetine in the treatment of attention-deficit/hyperactivity disorder: a head-to-head, randomised, double-blind, Phase IIIb study. CNS Drugs 2013;27:1081-1092. doi: 10.1007/s40263-013-0104-8 ClinicalTrials.gov: NCT01106430.Expert Opin Pharmacother. 2014 Sep;15(13):1961-5. doi: 10.1517/14656566.2014.934811. Epub 2014 Aug 1. Expert Opin Pharmacother. 2014. PMID: 25085429
-
Molecular Characterisation of the Mechanism of Action of Stimulant Drugs Lisdexamfetamine and Methylphenidate on ADHD Neurobiology: A Review.Neurol Ther. 2022 Dec;11(4):1489-1517. doi: 10.1007/s40120-022-00392-2. Epub 2022 Aug 11. Neurol Ther. 2022. PMID: 35951288 Free PMC article. Review.
-
Onset of efficacy of long-acting psychostimulants in pediatric attention-deficit/hyperactivity disorder.Postgrad Med. 2008 Sep;120(3):69-88. doi: 10.3810/pgm.2008.09.1909. Postgrad Med. 2008. PMID: 18824827 Review.
Cited by
-
A decision support system based on artificial intelligence and systems biology for the simulation of pancreatic cancer patient status.CPT Pharmacometrics Syst Pharmacol. 2023 Jul;12(7):916-928. doi: 10.1002/psp4.12961. Epub 2023 Mar 31. CPT Pharmacometrics Syst Pharmacol. 2023. PMID: 37002678 Free PMC article.
-
Computer-Aided Drug Design (CADD) to De-Orphanize Marine Molecules: Finding Potential Therapeutic Agents for Neurodegenerative and Cardiovascular Diseases.Mar Drugs. 2022 Jan 5;20(1):53. doi: 10.3390/md20010053. Mar Drugs. 2022. PMID: 35049908 Free PMC article.
-
Aflibercept Off-Target Effects in Diabetic Macular Edema: An In Silico Modeling Approach.Int J Mol Sci. 2024 Mar 23;25(7):3621. doi: 10.3390/ijms25073621. Int J Mol Sci. 2024. PMID: 38612432 Free PMC article.
-
In silico evaluation of the role of lisdexamfetamine on attention-deficit/hyperactivity disorder common psychiatric comorbidities: mechanistic insights on binge eating disorder and depression.Front Neurosci. 2023 Jun 30;17:1118253. doi: 10.3389/fnins.2023.1118253. eCollection 2023. Front Neurosci. 2023. PMID: 37457000 Free PMC article.
-
In silico clinical trial evaluating lisdexamfetamine's and methylphenidate's mechanism of action computational models in an attention-deficit/hyperactivity disorder virtual patients' population.Front Psychiatry. 2023 Jun 2;14:939650. doi: 10.3389/fpsyt.2023.939650. eCollection 2023. Front Psychiatry. 2023. PMID: 37333910 Free PMC article.
References
-
- Viceconti M, Henney A, Morley-Fletcher E. In silico clinical trials: how computer simulation will transform the biomedical industry. Int J Clin Trials. (2016) 3:37–46. 10.18203/2349-3259.ijct20161408 - DOI
LinkOut - more resources
Full Text Sources

