The Role of Melanocortin Plasticity in Pain-Related Outcomes After Alcohol Exposure
- PMID: 34803772
- PMCID: PMC8599269
- DOI: 10.3389/fpsyt.2021.764720
The Role of Melanocortin Plasticity in Pain-Related Outcomes After Alcohol Exposure
Abstract
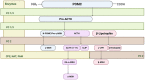
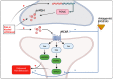
The global COVID-19 pandemic has shone a light on the rates and dangers of alcohol misuse in adults and adolescents in the US and globally. Alcohol exposure during adolescence causes persistent molecular, cellular, and behavioral changes that increase the risk of alcohol use disorder (AUD) into adulthood. It is established that alcohol abuse in adulthood increases the likelihood of pain hypersensitivity and the genesis of chronic pain, and humans report drinking alcohol to relieve pain symptoms. However, the longitudinal effects of alcohol exposure on pain and the underlying CNS signaling that mediates it are understudied. Specific brain regions mediate pain effects, alcohol effects, and pain-alcohol interactions, and neural signaling in those brain regions is modulated by neuropeptides. The CNS melanocortin system is sensitive to alcohol and modulates pain sensitivity, but this system is understudied in the context of pain-alcohol interactions. In this review, we focus on the role of melanocortin signaling in brain regions sensitive to alcohol and pain, in particular the amygdala. We also discuss interactions of melanocortins with other peptide systems, including the opioid system, as potential mediators of pain-alcohol interactions. Therapeutic strategies that target the melanocortin system may mitigate the negative consequences of alcohol misuse during adolescence and/or adulthood, including effects on pain-related outcomes.
Keywords: MC4R; alcohol; melanocortin; opioids; pain.
Copyright © 2021 Sharfman and Gilpin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Central Amygdala Circuits Mediate Hyperalgesia in Alcohol-Dependent Rats.J Neurosci. 2018 Sep 5;38(36):7761-7773. doi: 10.1523/JNEUROSCI.0483-18.2018. Epub 2018 Jul 27. J Neurosci. 2018. PMID: 30054393 Free PMC article.
-
Alcohol and Opioid Use, Co-Use, and Chronic Pain in the Context of the Opioid Epidemic: A Critical Review.Alcohol Clin Exp Res. 2018 Mar;42(3):478-488. doi: 10.1111/acer.13594. Epub 2018 Feb 6. Alcohol Clin Exp Res. 2018. PMID: 29314075 Free PMC article. Review.
-
Melanocortin activity in the amygdala influences alcohol intake.Pharmacol Biochem Behav. 2011 Mar;98(1):112-9. doi: 10.1016/j.pbb.2010.12.010. Epub 2010 Dec 15. Pharmacol Biochem Behav. 2011. PMID: 21167196
-
Activation of Melanocortin-4 Receptor Inhibits Both Neuroinflammation Induced by Early Exposure to Ethanol and Subsequent Voluntary Alcohol Intake in Adulthood in Animal Models: Is BDNF the Key Mediator?Front Cell Neurosci. 2020 Jan 28;14:5. doi: 10.3389/fncel.2020.00005. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32063838 Free PMC article.
-
The Role of the Melanocortin System in Drug and Alcohol Abuse.Int Rev Neurobiol. 2017;136:121-150. doi: 10.1016/bs.irn.2017.06.009. Epub 2017 Aug 14. Int Rev Neurobiol. 2017. PMID: 29056149 Review.
Cited by
-
Within and Beyond the Binary: Sex and Gender Differences in Pain and Alcohol Use Disorder.Curr Addict Rep. 2024 Feb;11(1):68-80. doi: 10.1007/s40429-023-00534-y. Epub 2023 Dec 22. Curr Addict Rep. 2024. PMID: 40747361 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

