Prospective Quantitative Neuroimaging Analysis of Putative Temporal Lobe Epilepsy
- PMID: 34803885
- PMCID: PMC8602195
- DOI: 10.3389/fneur.2021.747580
Prospective Quantitative Neuroimaging Analysis of Putative Temporal Lobe Epilepsy
Abstract
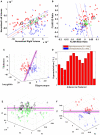
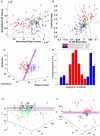
Purpose: A prospective study of individual and combined quantitative imaging applications for lateralizing epileptogenicity was performed in a cohort of consecutive patients with a putative diagnosis of mesial temporal lobe epilepsy (mTLE). Methods: Quantitative metrics were applied to MRI and nuclear medicine imaging studies as part of a comprehensive presurgical investigation. The neuroimaging analytics were conducted remotely to remove bias. All quantitative lateralizing tools were trained using a separate dataset. Outcomes were determined after 2 years. Of those treated, some underwent resection, and others were implanted with a responsive neurostimulation (RNS) device. Results: Forty-eight consecutive cases underwent evaluation using nine attributes of individual or combinations of neuroimaging modalities: 1) hippocampal volume, 2) FLAIR signal, 3) PET profile, 4) multistructural analysis (MSA), 5) multimodal model analysis (MMM), 6) DTI uncertainty analysis, 7) DTI connectivity, and 9) fMRI connectivity. Of the 24 patients undergoing resection, MSA, MMM, and PET proved most effective in predicting an Engel class 1 outcome (>80% accuracy). Both hippocampal volume and FLAIR signal analysis showed 76% and 69% concordance with an Engel class 1 outcome, respectively. Conclusion: Quantitative multimodal neuroimaging in the context of a putative mTLE aids in declaring laterality. The degree to which there is disagreement among the various quantitative neuroimaging metrics will judge whether epileptogenicity can be confined sufficiently to a particular temporal lobe to warrant further study and choice of therapy. Prediction models will improve with continued exploration of combined optimal neuroimaging metrics.
Keywords: MRI; lateralization; multimodal analysis; neuroimaging; temporal lobe epilepsy.
Copyright © 2021 Elisevich, Davoodi-Bojd, Heredia and Soltanian-Zadeh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

