Sinomenine Relieves Airway Remodeling By Inhibiting Epithelial-Mesenchymal Transition Through Downregulating TGF-β1 and Smad3 Expression In Vitro and In Vivo
- PMID: 34804018
- PMCID: PMC8602849
- DOI: 10.3389/fimmu.2021.736479
Sinomenine Relieves Airway Remodeling By Inhibiting Epithelial-Mesenchymal Transition Through Downregulating TGF-β1 and Smad3 Expression In Vitro and In Vivo
Abstract
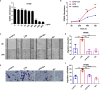
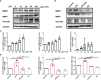
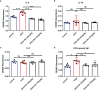
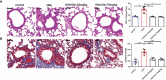
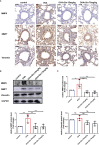
Airway remodeling is associated with dysregulation of epithelial-mesenchymal transition (EMT) in patients with asthma. Sinomenine (Sin) is an effective, biologically active alkaloid that has been reported to suppress airway remodeling in mice with asthma. However, the molecular mechanisms behind this effect remain unclear. We aimed to explore the potential relationship between Sin and EMT in respiratory epithelial cells in vitro and in vivo. First, 16HBE cells were exposed to 100 μg/mL LPS and treated with 200 μg/mL Sin. Cell proliferation, migration, and wound healing assays were performed to evaluate EMT, and EMT-related markers were detected using Western blotting. Mice with OVA-induced asthma were administered 35 mg/kg or 75 mg/kg Sin. Airway inflammation and remodeling detection experiments were performed, and EMT-related factors and proteins in the TGF-β1 pathway were detected using IHC and Western blotting. We found that Sin suppressed cell migration but not proliferation in LPS-exposed 16HBE cells. Sin also inhibited MMP7, MMP9, and vimentin expression in 16HBE cells and respiratory epithelial cells from mice with asthma. Furthermore, it decreased OVA-specific IgE and IL-4 levels in serum, relieved airway remodeling, attenuated subepithelial collagen deposition, and downregulating TGF-β1and Smad3 expression in mice with asthma. Our results suggest that Sin suppresses EMT by inhibiting IL-4 and downregulating TGF-β1 and Smad3 expression.
Keywords: EMT; Sinomenine; TGF-β1/Smad3 expression; airway remodeling; asthma.
Copyright © 2021 He, Cao, Wang, Wang, Miao, Li and Miao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

