Understanding the Secret of SARS-CoV-2 Variants of Concern/Interest and Immune Escape
- PMID: 34804024
- PMCID: PMC8602852
- DOI: 10.3389/fimmu.2021.744242
Understanding the Secret of SARS-CoV-2 Variants of Concern/Interest and Immune Escape
Abstract
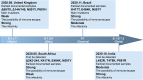
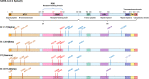
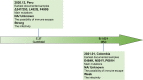
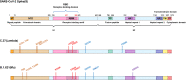
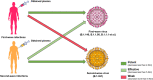
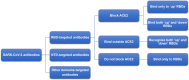
The global pandemic of the coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), places a heavy burden on global public health. Four SARS-CoV-2 variants of concern including B.1.1.7, B.1.351, B.1.617.2, and P.1, and two variants of interest including C.37 and B.1.621 have been reported to have potential immune escape, and one or more mutations endow them with worrisome epidemiologic, immunologic, or pathogenic characteristics. This review introduces the latest research progress on SARS-CoV-2 variants of interest and concern, key mutation sites, and their effects on virus infectivity, mortality, and immune escape. Moreover, we compared the effects of various clinical SARS-CoV-2 vaccines and convalescent sera on epidemic variants, and evaluated the neutralizing capability of several antibodies on epidemic variants. In the end, SARS-CoV-2 evolution strategies in different transmission stages, the impact of different vaccination strategies on SARS-CoV-2 immune escape, antibody therapy strategies and COVID-19 epidemic control prospects are discussed. This review will provide a systematic and comprehensive understanding of the secret of SARS-CoV-2 variants of interest/concern and immune escape.
Keywords: SARS-CoV-2 variants; immune escape; neutralizing antibody; vaccine; variants of concern; variants of interest.
Copyright © 2021 Lou, Li, Pang, Jiang, Guan, Tian, Hu, Fan and Fan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

